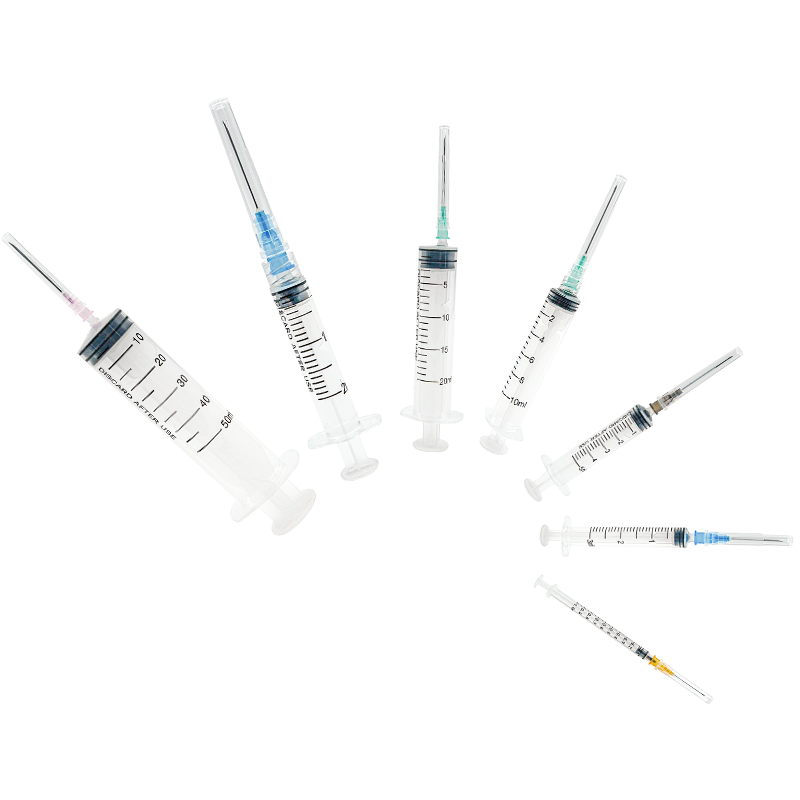
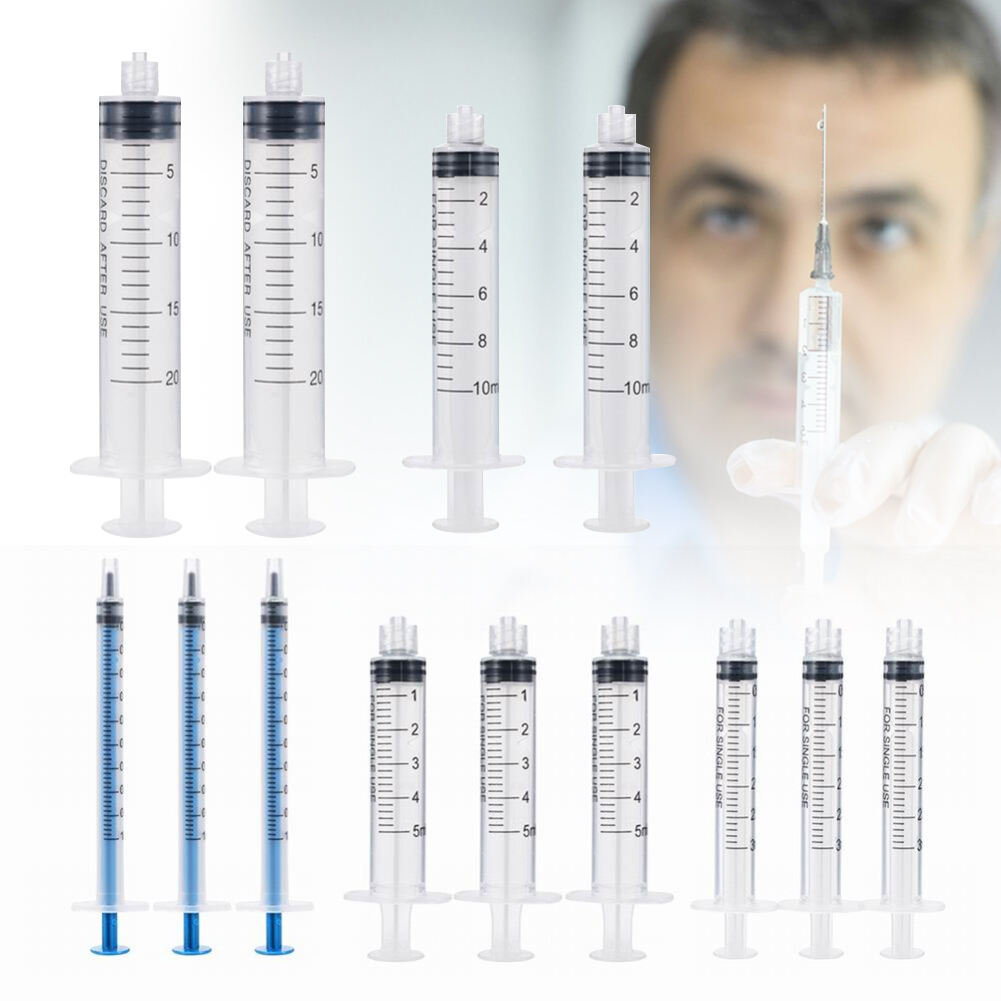
5ml plastic syringe manufacturer
As a leading 5ml plastic syringe manufacturer, our facility combines cutting-edge automation technology with stringent quality control processes to produce medical-grade syringes that meet international standards. Our manufacturing capabilities include state-of-the-art clean rooms, automated assembly lines, and advanced injection molding systems that ensure precise component production. The facility specializes in producing 5ml syringes with features such as clear barrel markings, smooth plunger action, and secure luer lock connections. We implement a comprehensive quality management system that covers raw material testing, in-process checks, and final product validation. Our manufacturing process adheres to ISO 13485 standards and FDA regulations, guaranteeing consistent product quality and safety. The facility's production capacity exceeds 10 million units monthly, with flexibility to scale according to demand. We utilize medical-grade polypropylene and maintain strict environmental controls throughout the manufacturing process. Our syringes undergo rigorous testing for sterility, pyrogenicity, and mechanical functionality, ensuring they meet healthcare providers' exacting requirements. The facility also maintains full traceability systems and documentation for each production batch, supporting regulatory compliance and patient safety.