5ml luer lock syringe manufacturer
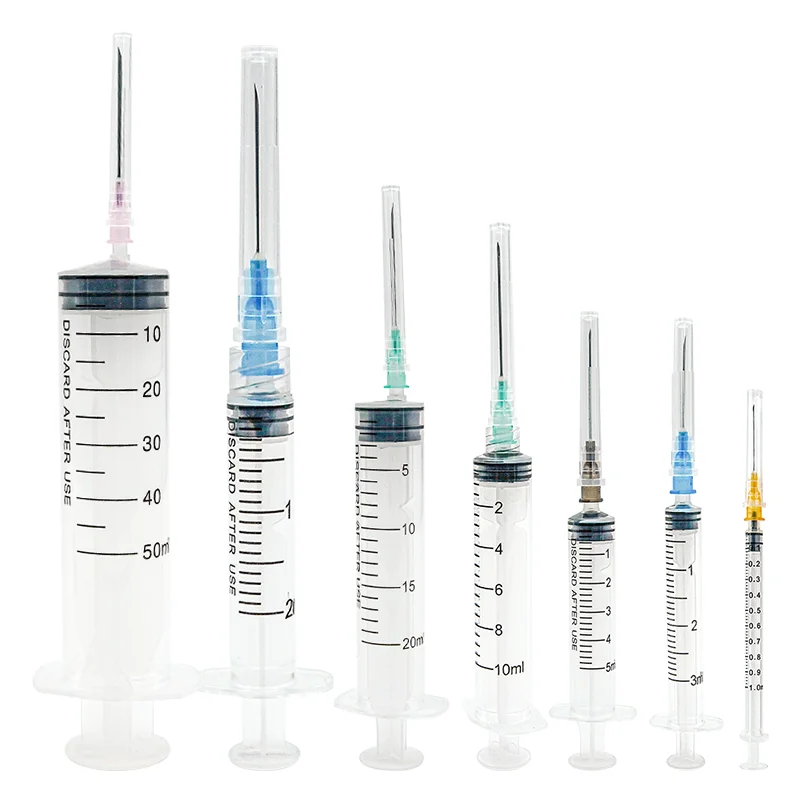
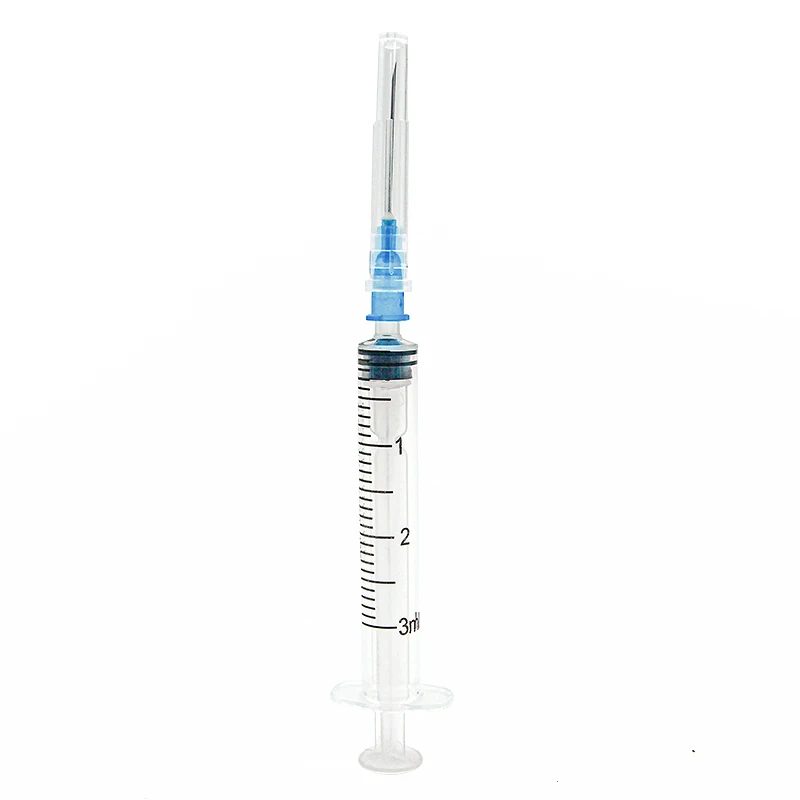
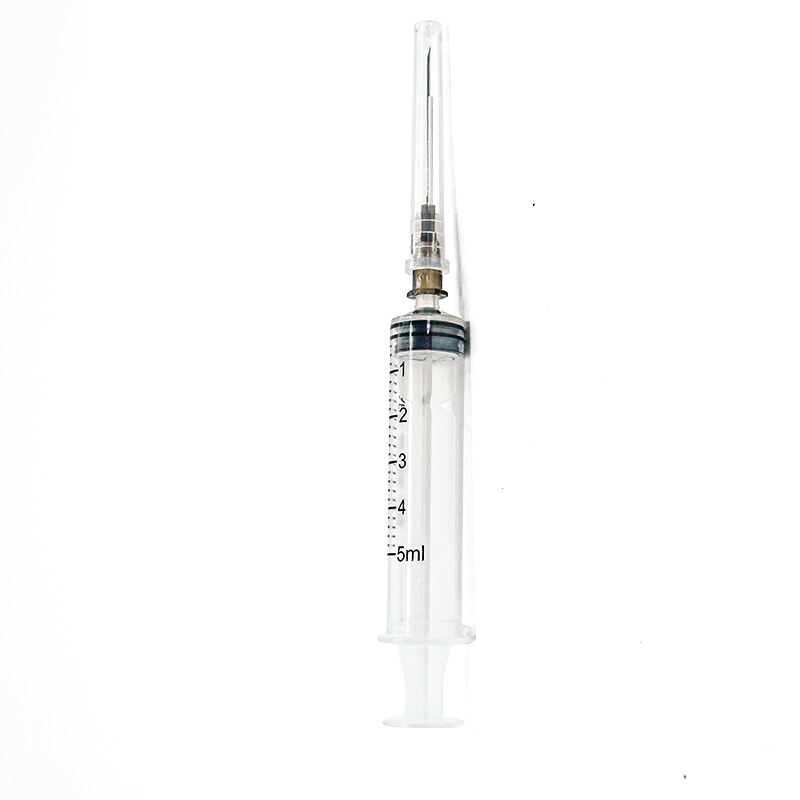
A 5ml luer lock syringe manufacturer stands at the forefront of medical device production, specializing in the precise engineering and manufacturing of essential medical instruments. These manufacturers employ state of the art facilities equipped with advanced injection molding technology and automated assembly lines to produce high quality syringes that meet international standards. The manufacturing process incorporates medical grade materials, primarily polypropylene and polyethylene, ensuring biocompatibility and chemical resistance. Quality control measures include rigorous testing for sterility, leak prevention, and dimensional accuracy. The facility maintains ISO 13485 certification and follows Good Manufacturing Practice guidelines, guaranteeing consistent product quality. The manufacturing capabilities extend to various components including the barrel, plunger, and the distinctive luer lock mechanism, which provides secure connections to needles and other medical devices. The production line implements automated vision systems for defect detection and maintains cleanroom conditions to prevent contamination. These manufacturers also offer customization options for different medical applications, from laboratory research to clinical use, while maintaining strict adherence to regulatory requirements and safety standards.