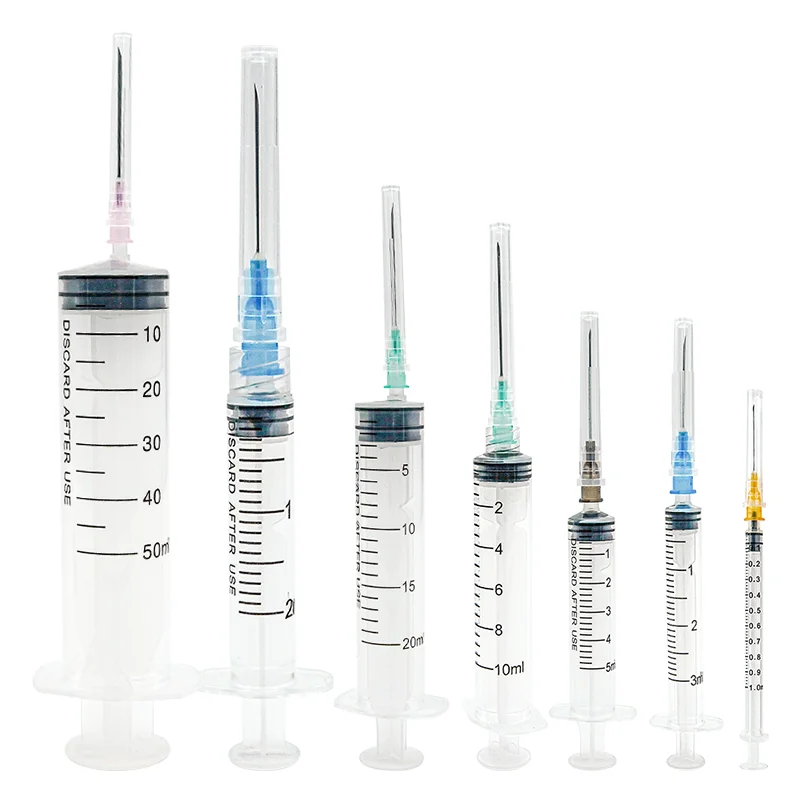
Comprehensive Regulatory Compliance
The manufacturer maintains strict adherence to international regulatory standards, including FDA, CE, and ISO requirements. Their quality management system includes regular internal audits and third-party certifications to ensure continuous compliance. The facility maintains detailed documentation of all manufacturing processes, material specifications, and quality control measures. Their regulatory team stays current with changing requirements and implements necessary updates to manufacturing processes. The manufacturer provides comprehensive regulatory documentation packages to support client registration requirements in different markets. Their compliance program includes regular staff training on GMP requirements and updated regulatory standards. The facility undergoes regular inspections by regulatory authorities and maintains excellent compliance records.