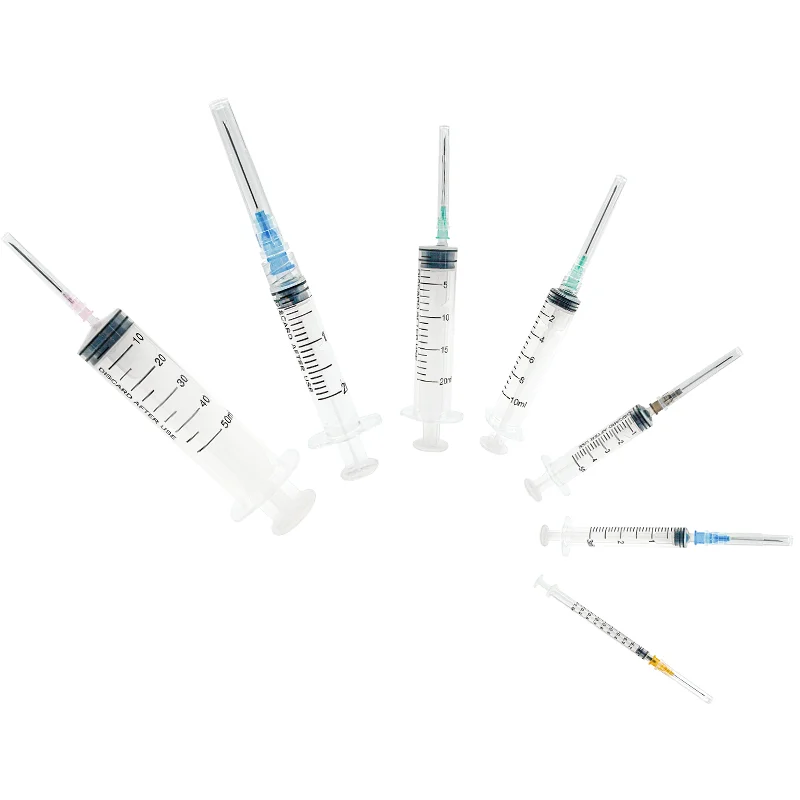
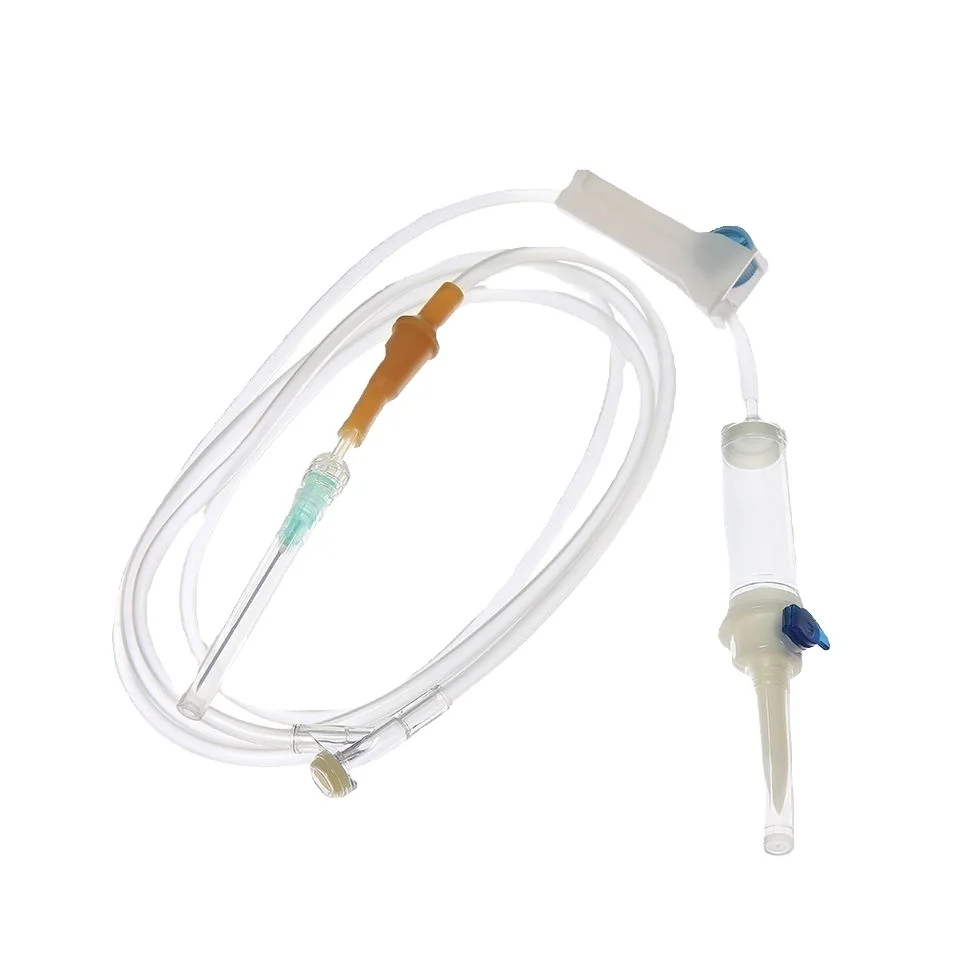
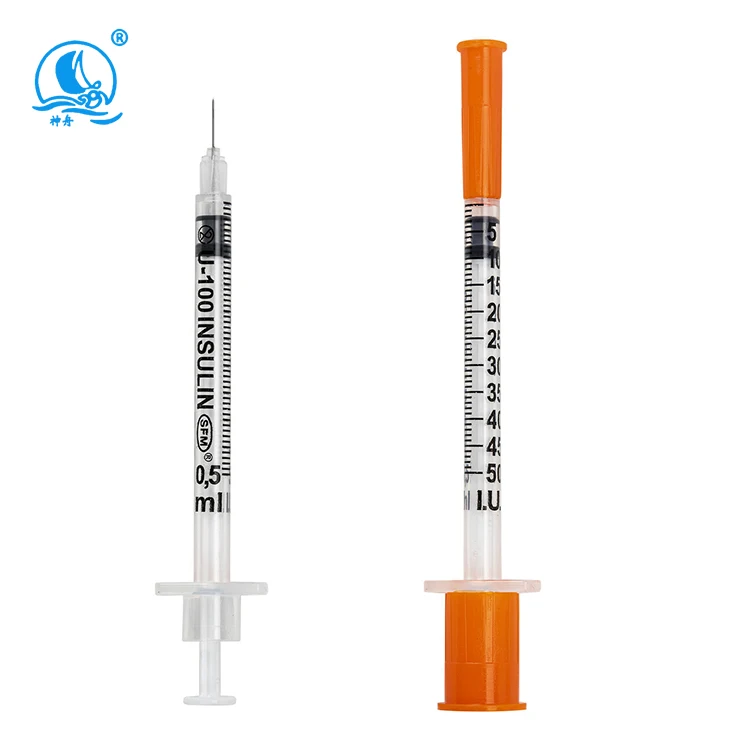
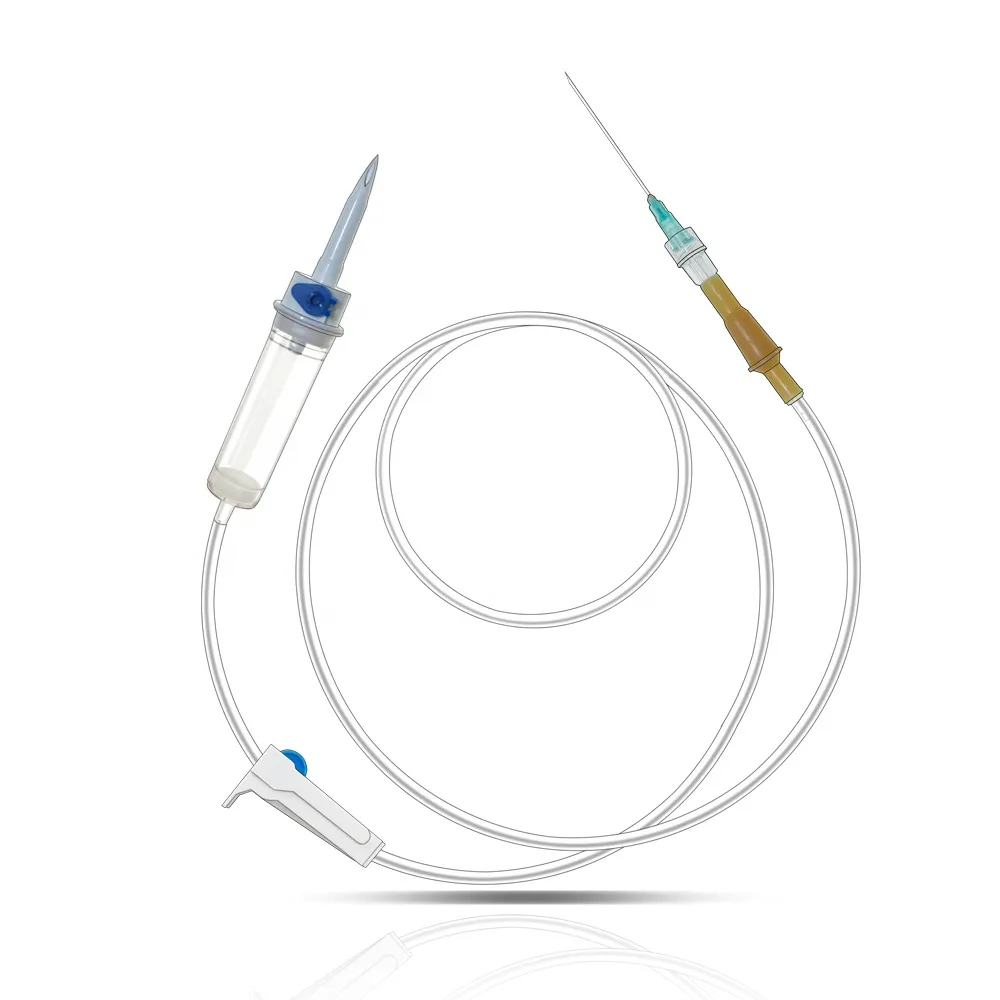
needle 23 manufacturer
The needle 23 manufacturer stands at the forefront of precision medical device production, specializing in the creation of high-quality hypodermic needles that meet stringent industry standards. This state-of-the-art facility combines advanced automation technology with meticulous quality control processes to produce consistent, reliable needles for medical applications. The manufacturing process incorporates cutting-edge materials and sophisticated engineering techniques to ensure optimal needle sharpness, durability, and patient comfort. The facility's production lines are equipped with automated inspection systems that verify each needle's dimensions, including wall thickness, point geometry, and overall length, maintaining accuracy to within micrometers. Environmental controls within the manufacturing space maintain pristine conditions, with HEPA filtration systems and positive air pressure to prevent contamination. The manufacturer's commitment to innovation is evident in their research and development department, which continuously works to improve needle design and manufacturing processes. Their comprehensive quality management system ensures compliance with ISO 13485 standards and FDA regulations, guaranteeing the safety and reliability of every needle produced. The facility's production capacity can meet high-volume demands while maintaining consistent quality across all batches.