medical injection syringe manufacturer
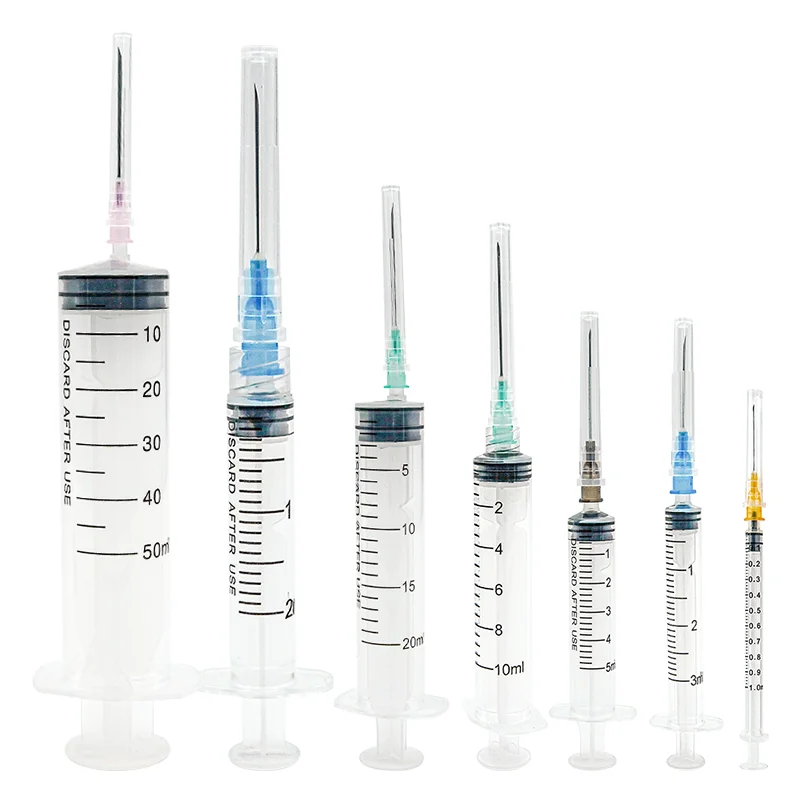
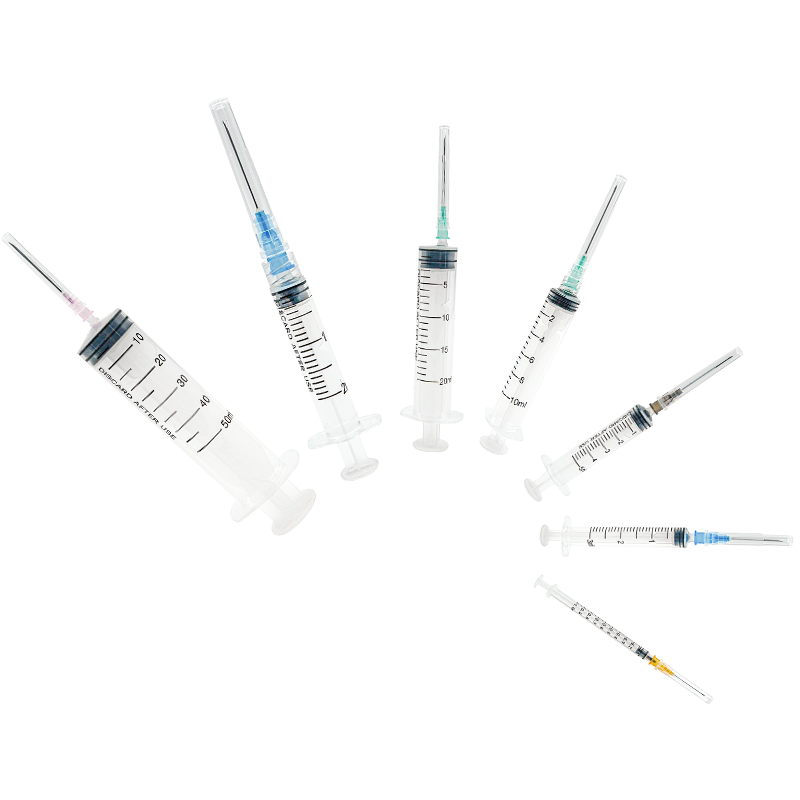
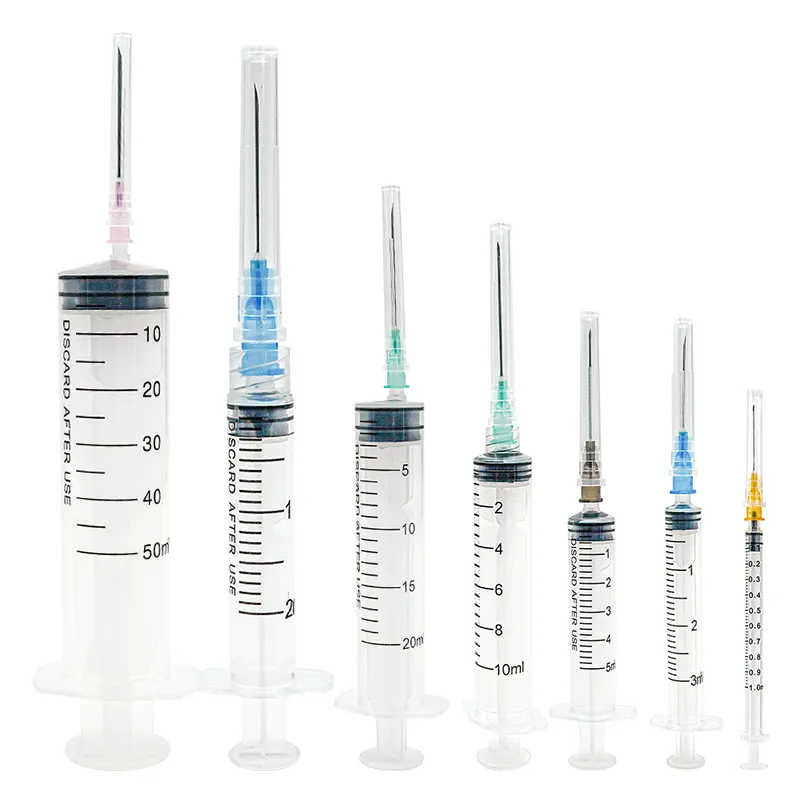
A medical injection syringe manufacturer stands as a cornerstone in modern healthcare manufacturing, specializing in the production of high-precision medical devices essential for safe and accurate drug delivery. These facilities integrate advanced automation systems and cleanroom technology to ensure the highest standards of quality and sterility in production. The manufacturing process encompasses multiple stages, from raw material selection to final quality inspection, utilizing state-of-the-art equipment for precision molding, assembly, and sterilization. The facilities typically feature automated production lines capable of producing various syringe sizes ranging from 1ml to 60ml, with options for both luer lock and luer slip designs. Quality control measures include real-time monitoring systems, automated vision inspection, and rigorous testing protocols to ensure compliance with international medical device standards such as ISO 13485 and FDA regulations. The manufacturer's capabilities extend to producing specialized syringes for specific medical applications, including insulin delivery, vaccination programs, and precise medication administration in critical care settings. Environmental control systems maintain optimal temperature, humidity, and air quality throughout the production area, while advanced tracking systems ensure complete product traceability from manufacturing to distribution.