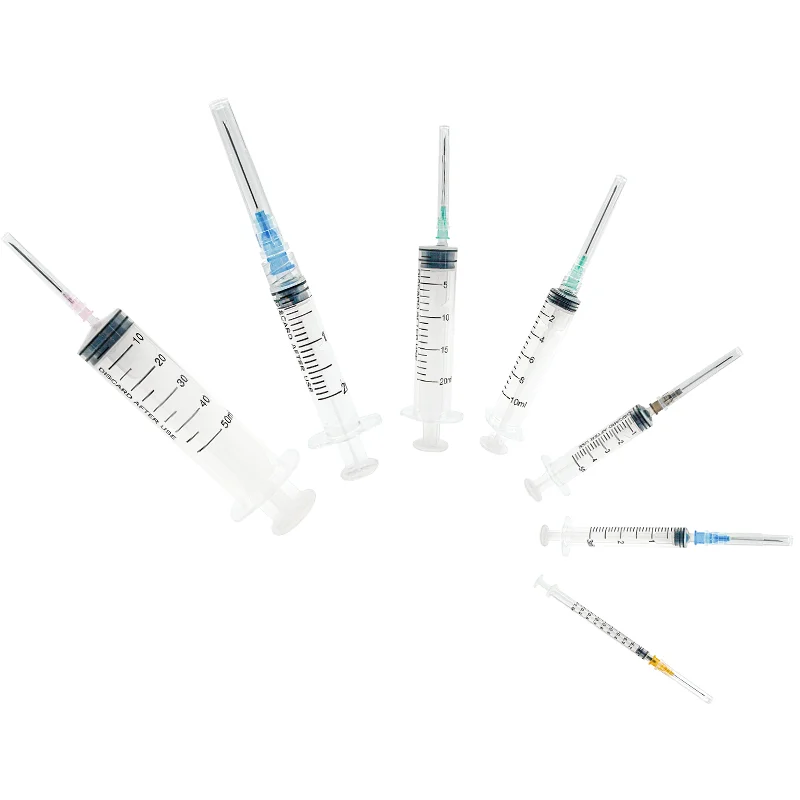
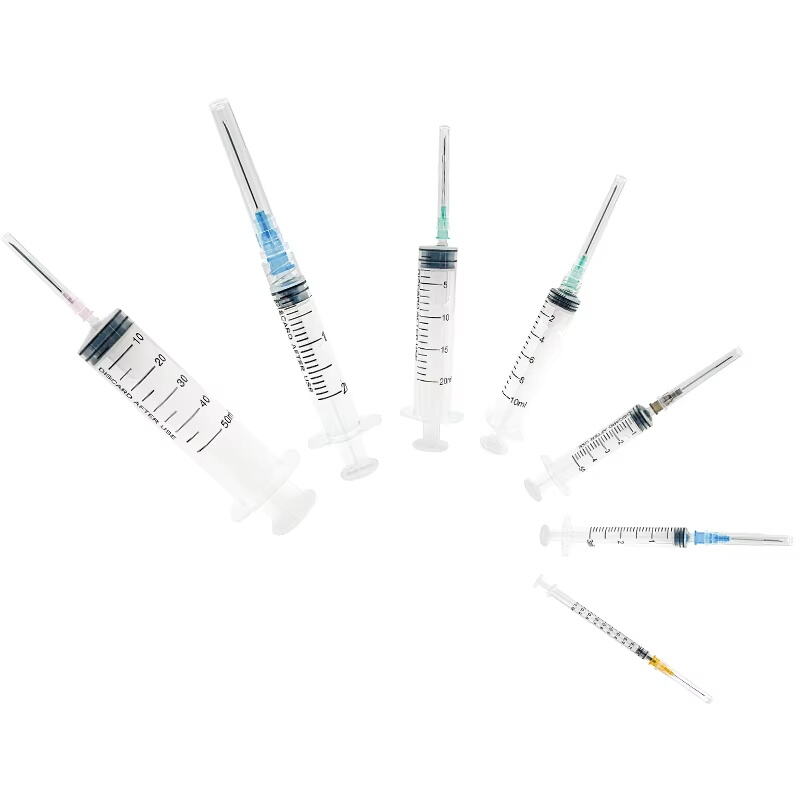
20 ml injection syringe manufacturer
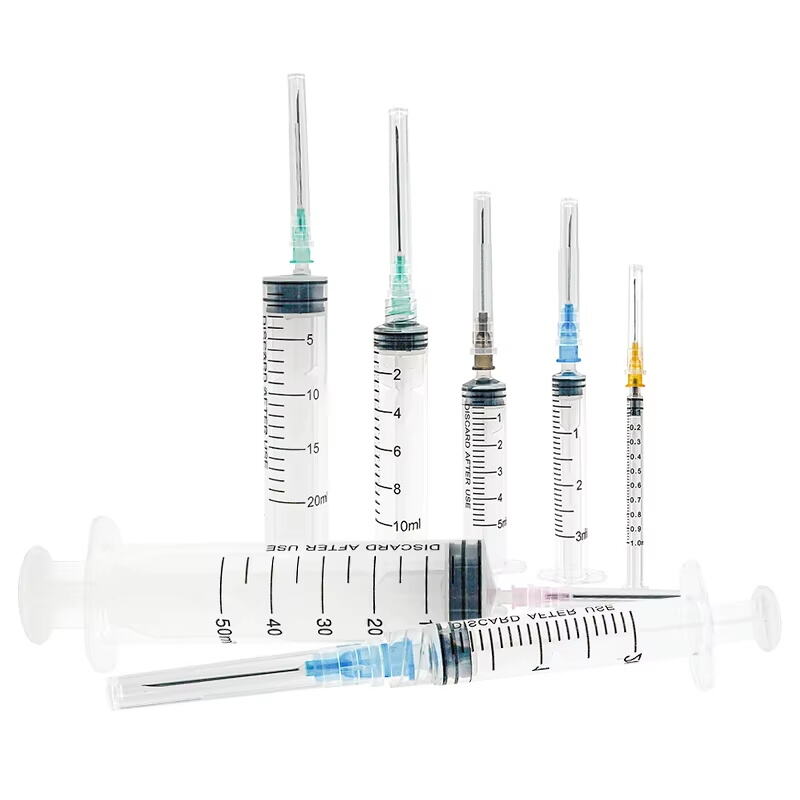
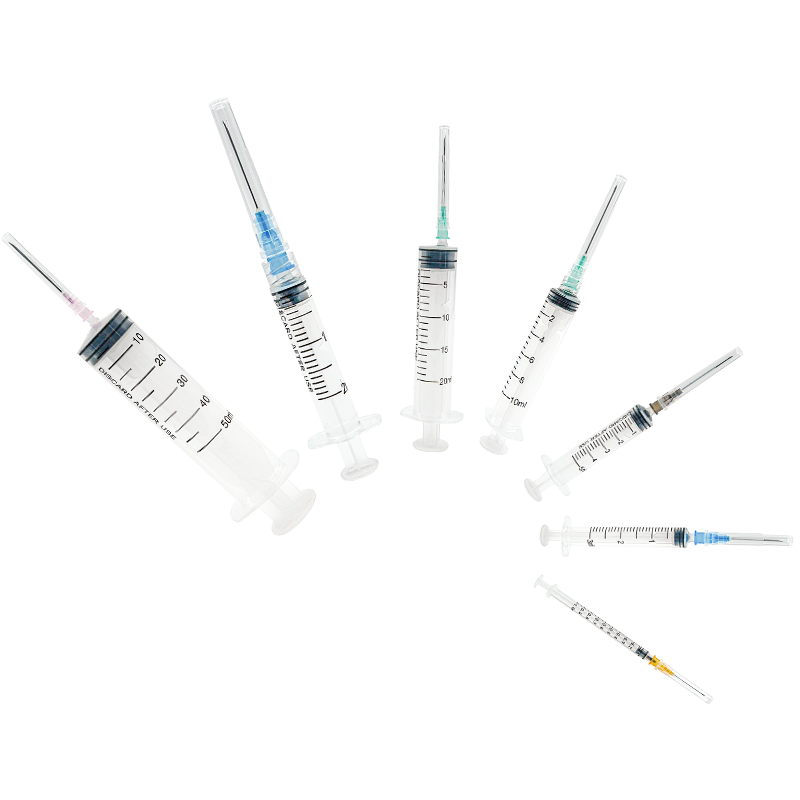
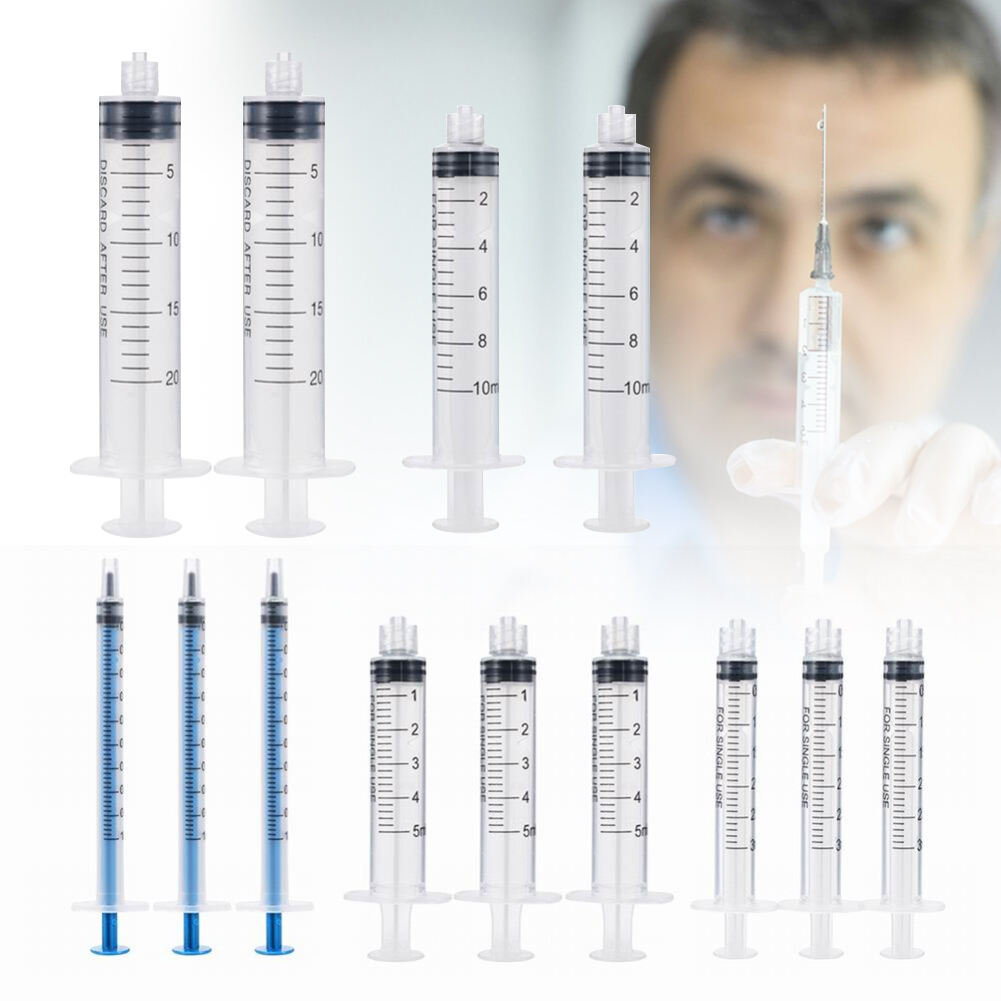
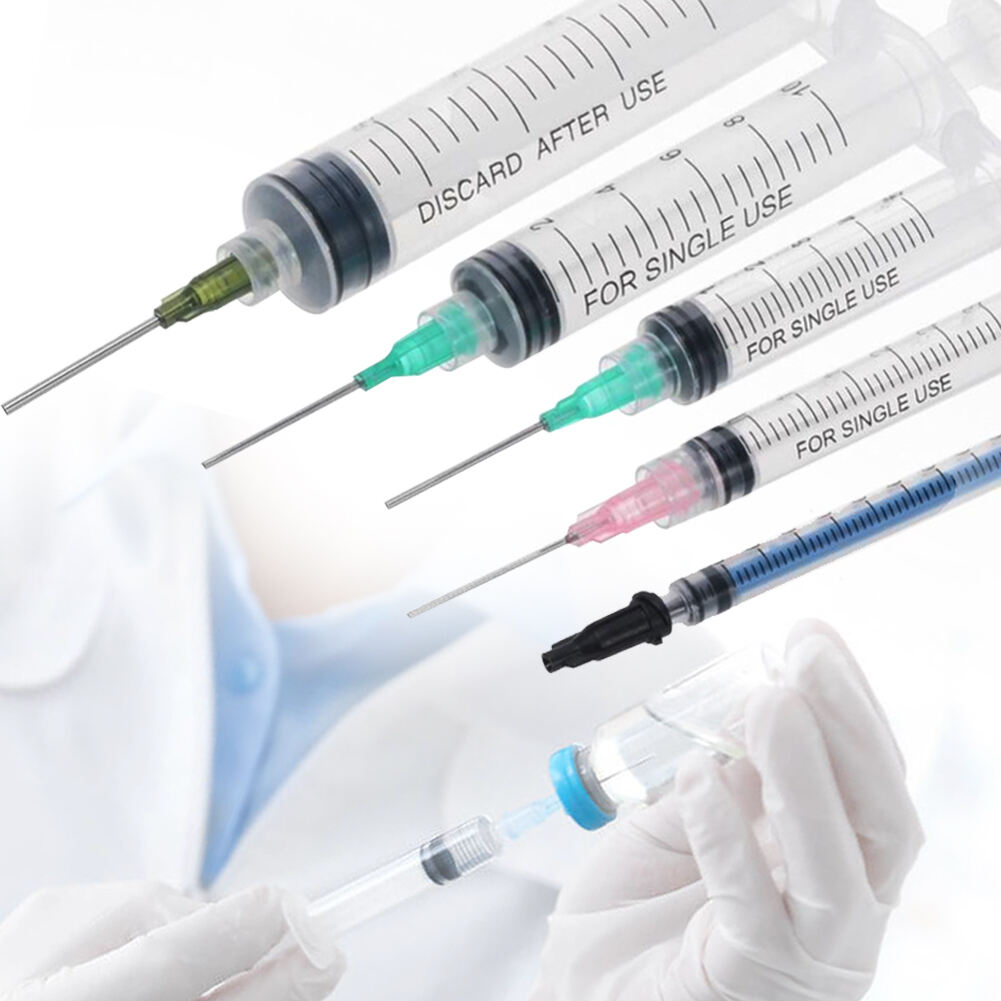
A 20 ml injection syringe manufacturer represents a cornerstone in medical device production, specializing in the creation of high-precision medical tools essential for healthcare delivery. These manufacturers employ state-of-the-art production facilities equipped with advanced injection molding technology and automated assembly lines to ensure consistent quality and sterility. The production process incorporates medical-grade materials, primarily polypropylene and polyethylene, which meet stringent international safety standards including ISO 13485 certification. The manufacturing facility maintains controlled environments with cleanroom technology, ensuring particles and contaminants are kept at minimal levels throughout production. Quality control measures include rigorous testing procedures, from raw material verification to final product inspection, utilizing sophisticated measurement tools and automated vision systems. The manufacturer's capabilities extend to various syringe designs, including Luer lock and Luer slip variants, with precise graduations and smooth plunger action. These facilities typically maintain high-volume production capabilities while ensuring each syringe meets exact specifications for capacity, accuracy, and safety features.