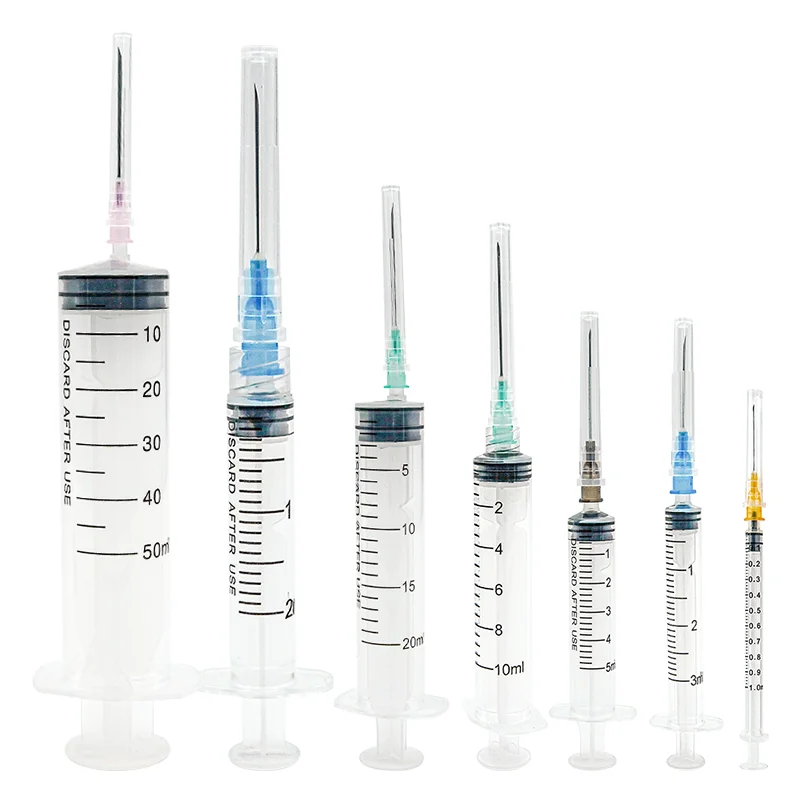
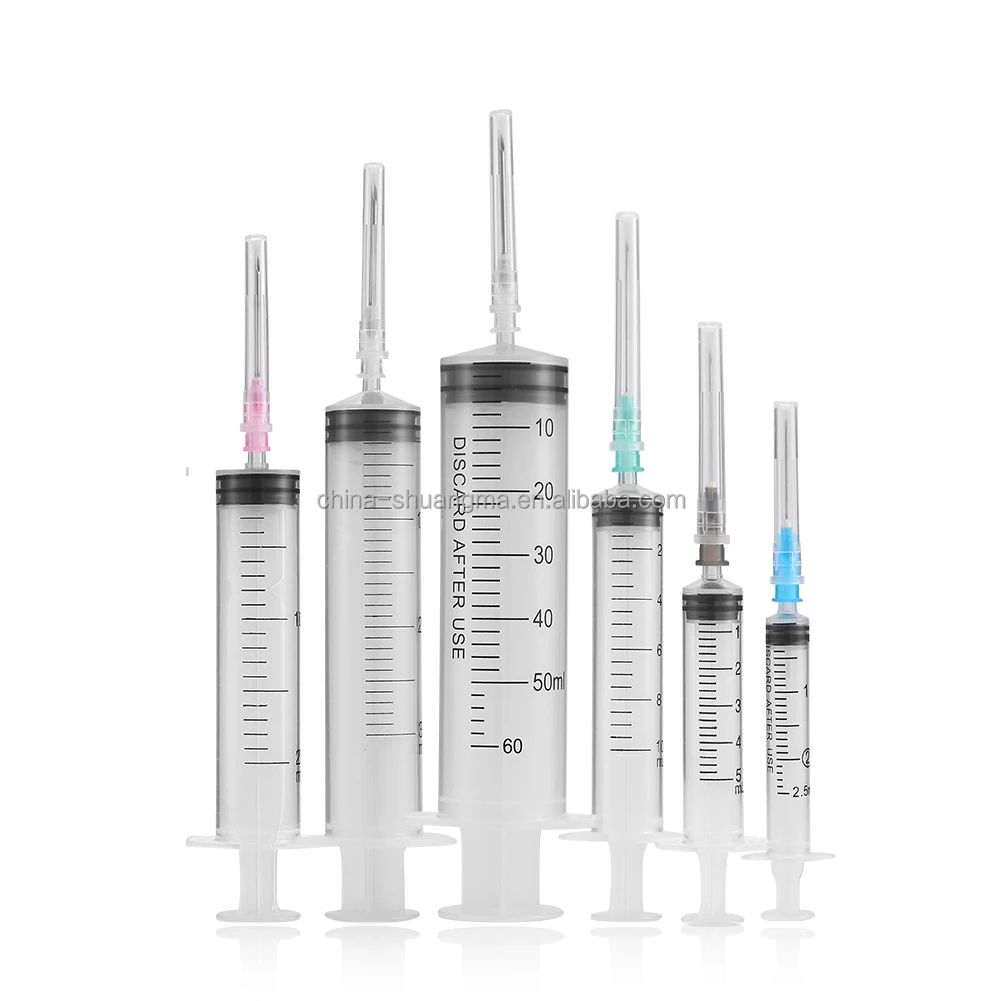
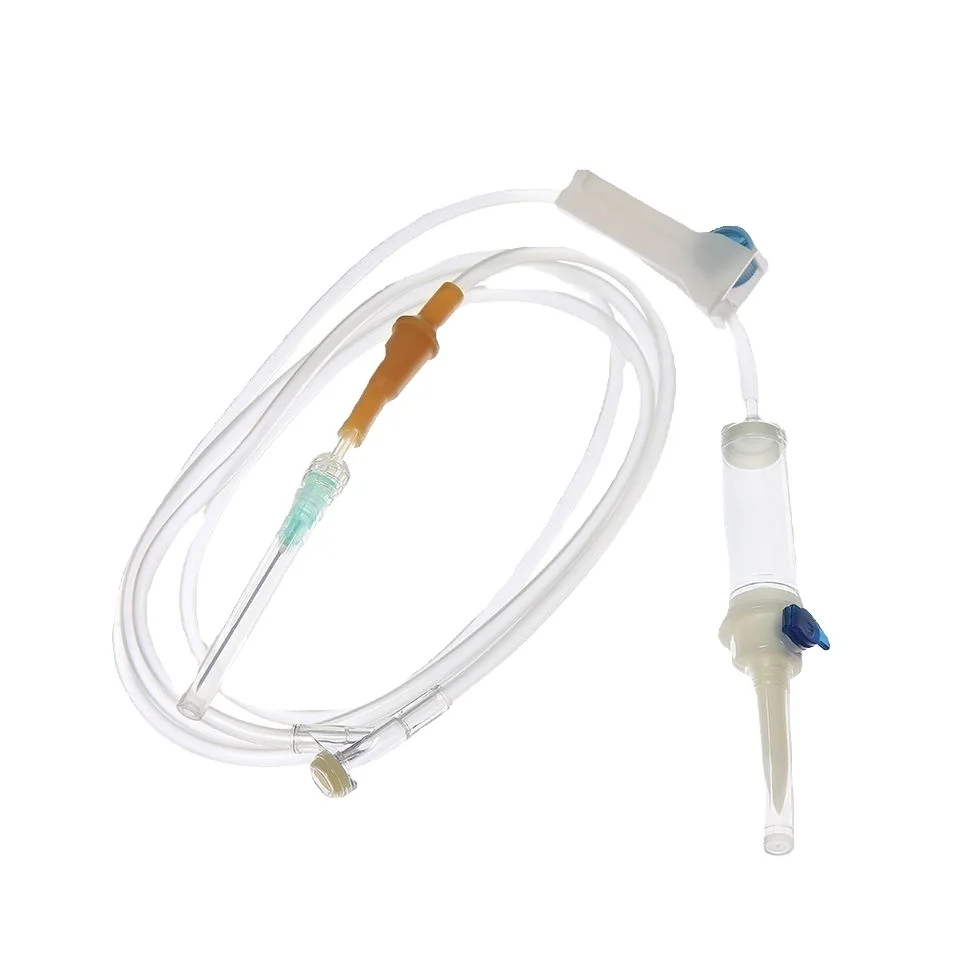
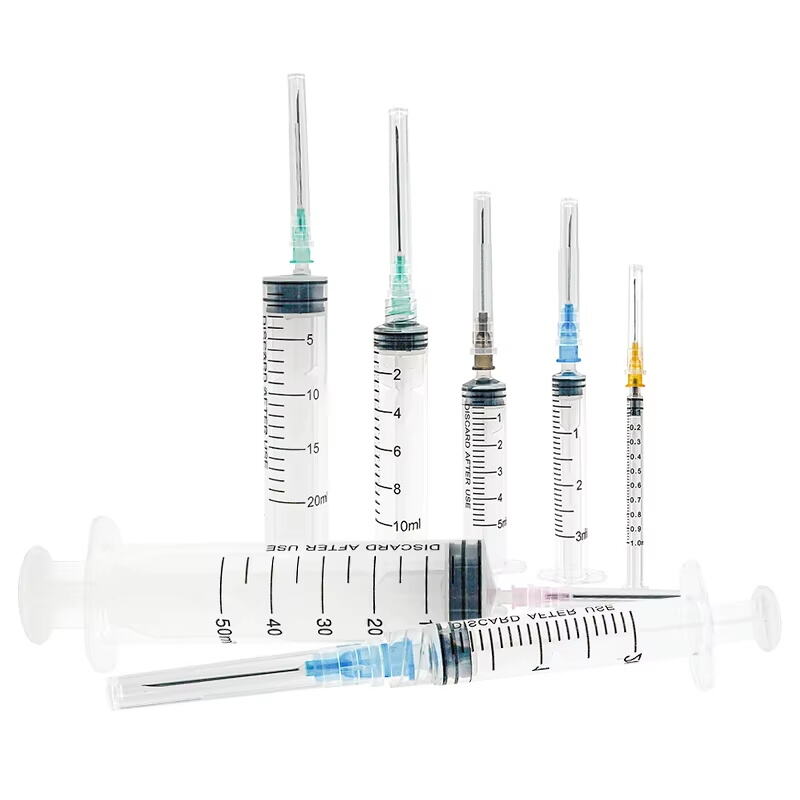
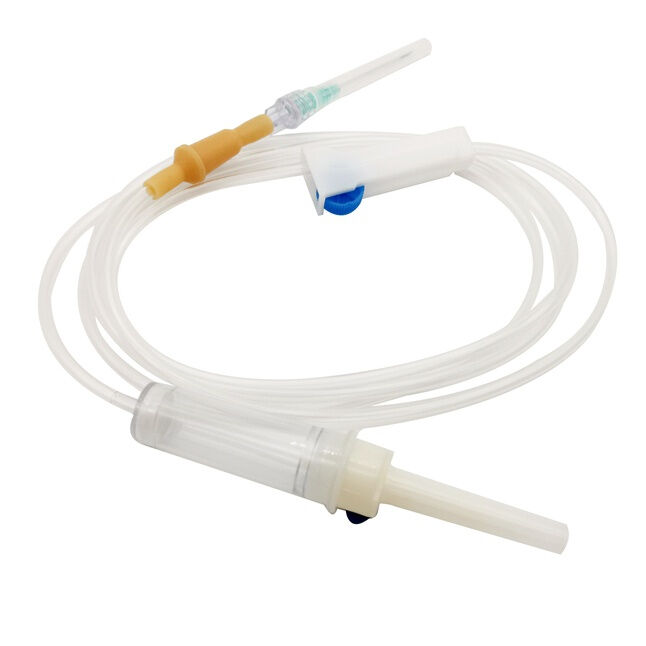
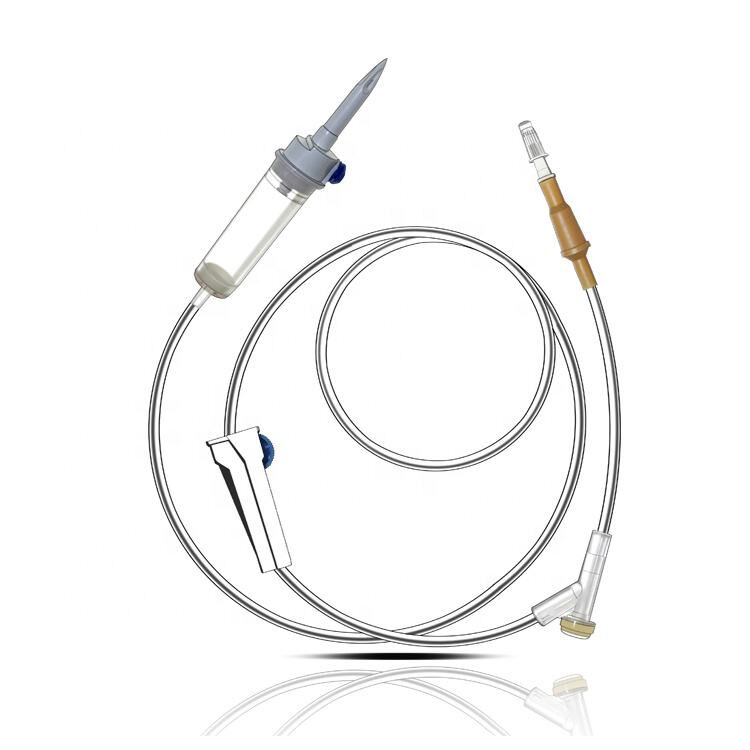
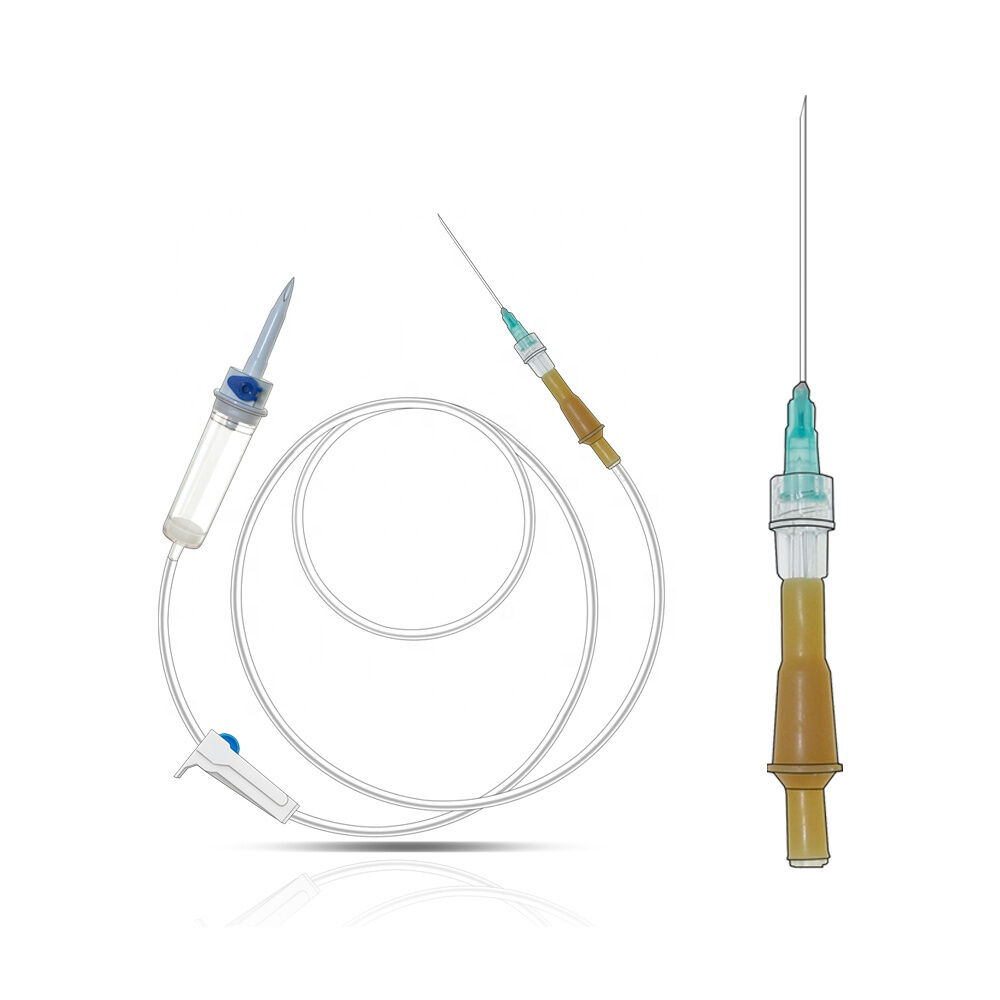
iv set infusion manufacturer
An IV set infusion manufacturer specializes in producing essential medical devices that deliver fluids, medications, and nutrients directly into patients' bloodstreams. These manufacturers employ state-of-the-art production facilities equipped with clean rooms and advanced quality control systems to ensure the highest standards of safety and reliability. Their manufacturing processes incorporate precision engineering to create sterile, medical-grade components including drip chambers, roller clamps, injection ports, and tubing sets. Modern IV set manufacturers utilize automated assembly lines and sophisticated testing equipment to maintain consistent product quality while meeting international regulatory requirements such as FDA and CE standards. They implement comprehensive quality management systems that cover everything from raw material selection to final product sterilization. The manufacturing facilities are designed to meet ISO 13485 medical device standards, ensuring that every IV set produced meets strict quality benchmarks. These manufacturers also invest in research and development to improve product designs, enhance safety features, and develop innovative solutions for specific medical applications. They maintain robust documentation systems for traceability and regulatory compliance while providing technical support and training resources for healthcare providers.