disposable syringe 10 ml manufacturer
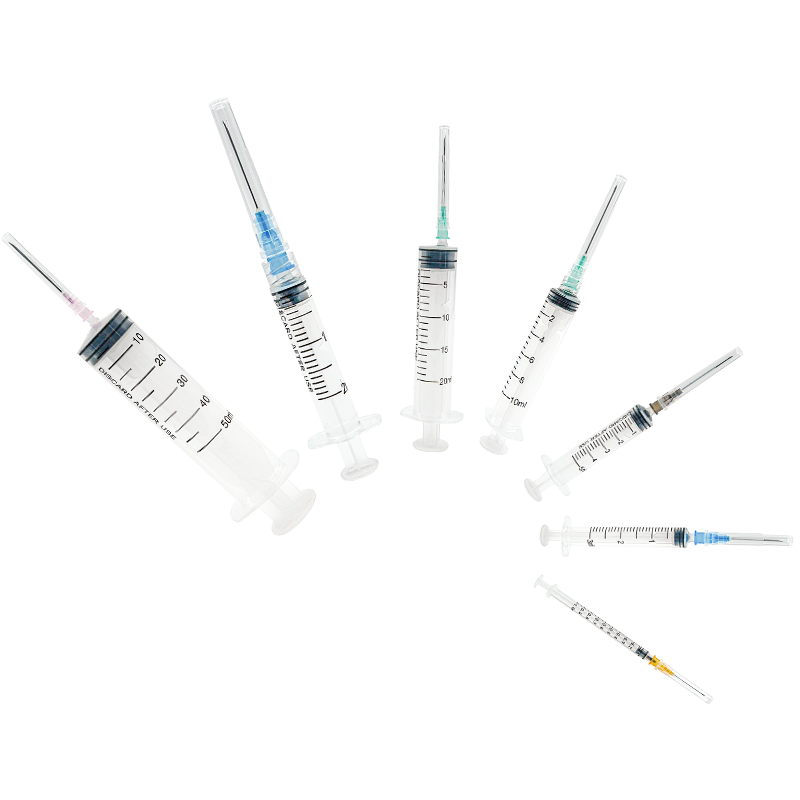
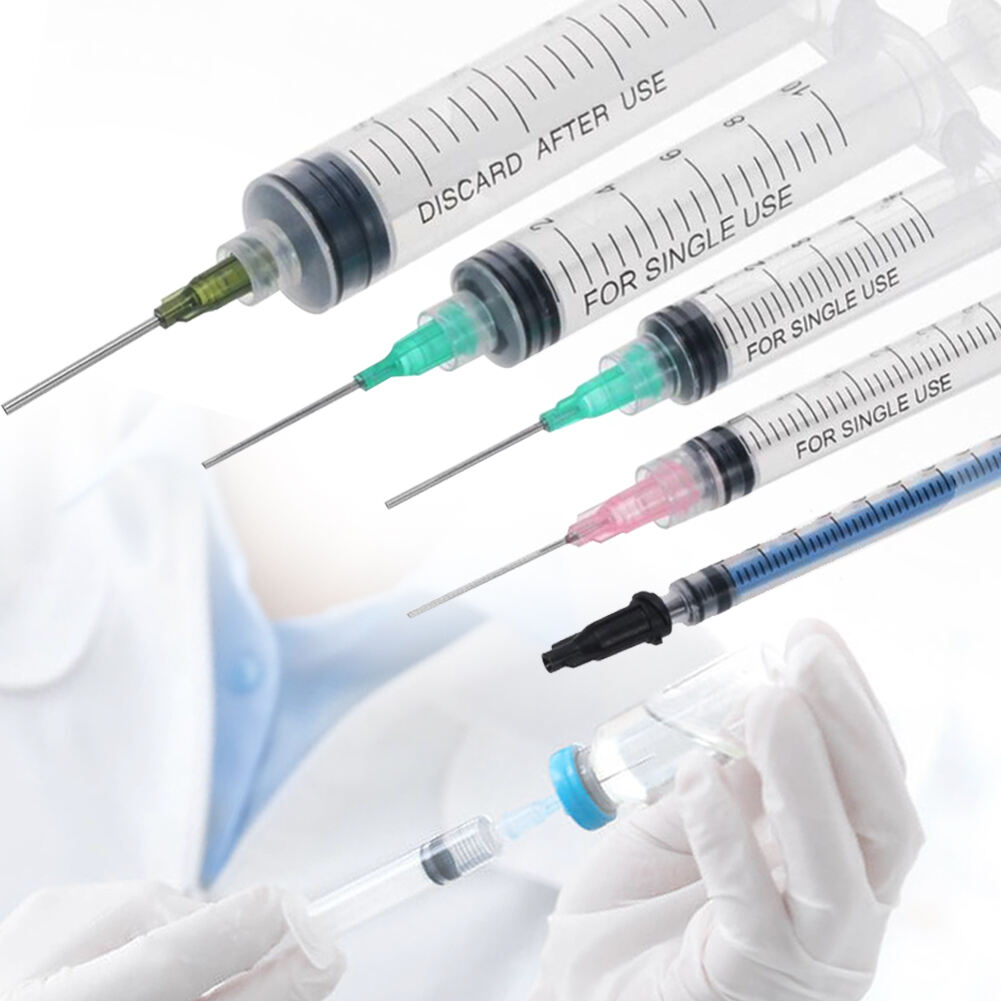
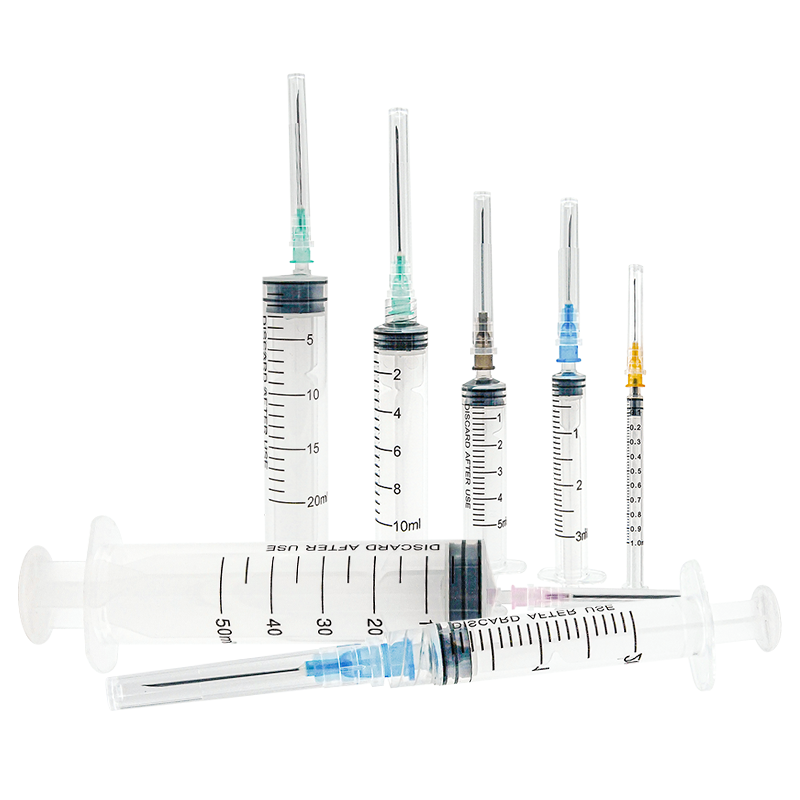
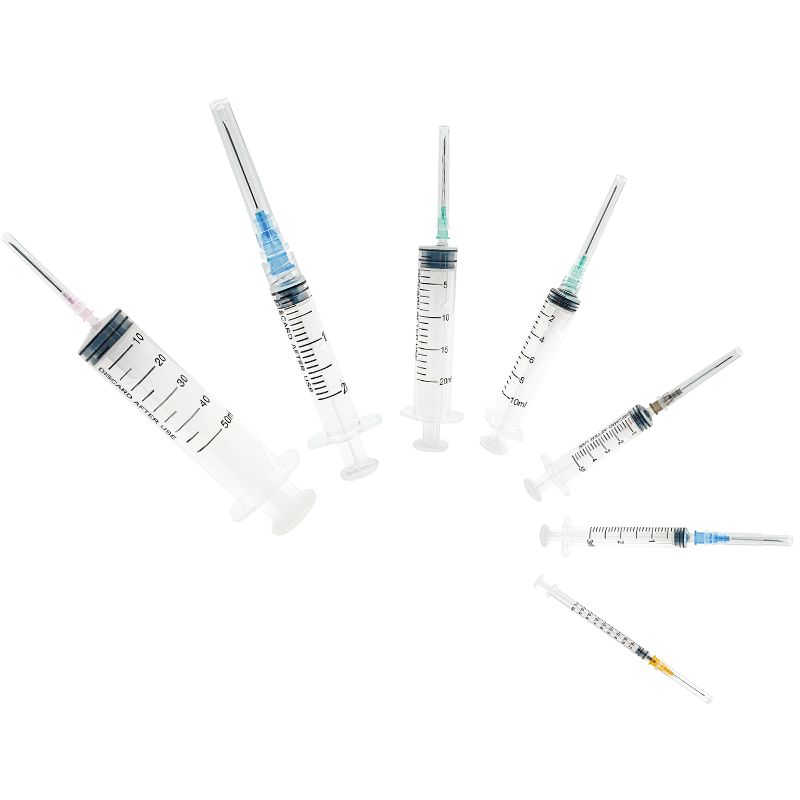
A disposable syringe 10 ml manufacturer specializes in producing high-quality, single-use medical devices essential for healthcare settings worldwide. These manufacturers employ advanced injection molding technology and automated assembly lines to ensure precise production of sterile syringes. The manufacturing process involves multiple quality control checkpoints, from raw material testing to final product inspection, ensuring compliance with international standards such as ISO 13485 and FDA regulations. The production facility typically features cleanroom environments meeting ISO Class 7 or higher specifications, where syringes are manufactured using medical-grade polypropylene and high-quality rubber materials for the plunger. The 10 ml syringes are designed with clear barrel markings for accurate dosage measurement, smooth plunger action for controlled delivery, and secure luer lock or slip tip connections. These manufacturers also implement strict sterilization protocols, usually using ethylene oxide or gamma radiation, to ensure product safety. Advanced packaging solutions are employed to maintain sterility throughout storage and transportation, with each syringe individually wrapped and clearly labeled with essential information including lot numbers and expiration dates.