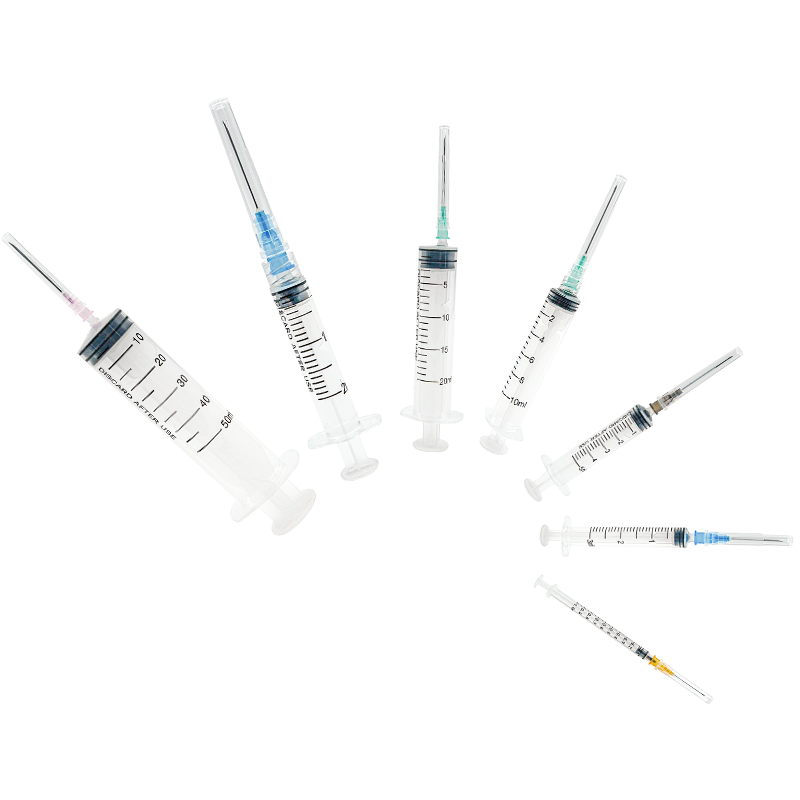
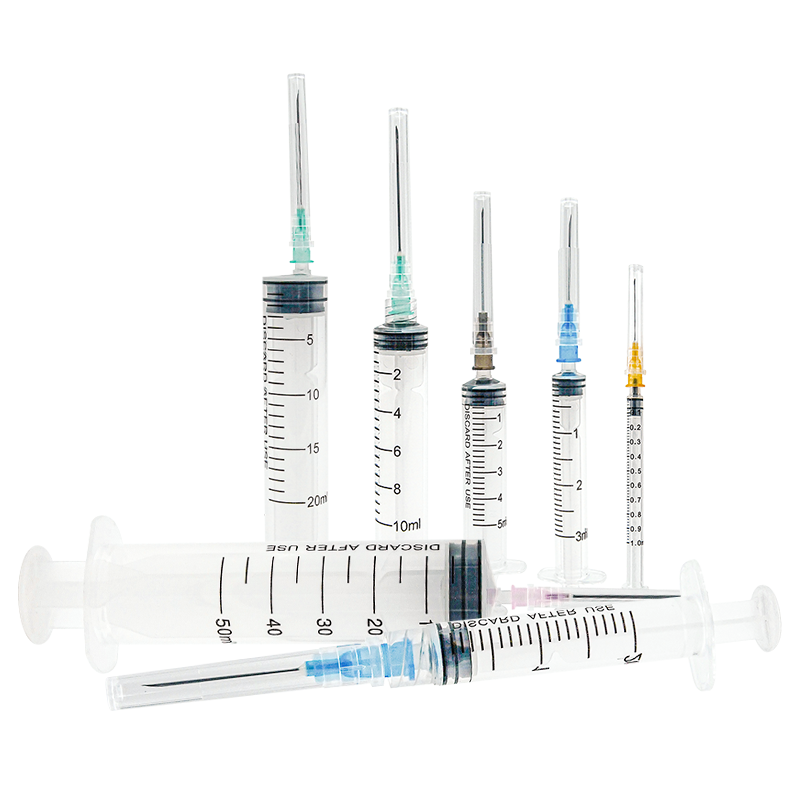
50 ml injection syringe manufacturer
As a leading 50 ml injection syringe manufacturer, our company stands at the forefront of medical device production, combining cutting-edge technology with precision engineering. Our state-of-the-art manufacturing facility employs advanced automation systems and strict quality control measures to produce high-quality 50 ml syringes that meet international standards. The manufacturing process incorporates medical-grade materials and innovative design features, ensuring optimal performance and patient safety. Our facility utilizes clean room technology and automated assembly lines to maintain consistent product quality and minimize contamination risks. We implement rigorous testing protocols at every production stage, from raw material inspection to final product verification. The manufacturing capability extends to various syringe types, including luer lock and luer slip designs, with options for specialized coatings and custom graduations. Our production capacity reaches millions of units annually, supported by efficient inventory management and distribution systems. The facility maintains ISO 13485 certification and follows GMP guidelines, demonstrating our commitment to quality and regulatory compliance.