insulin injection syringe manufacturer
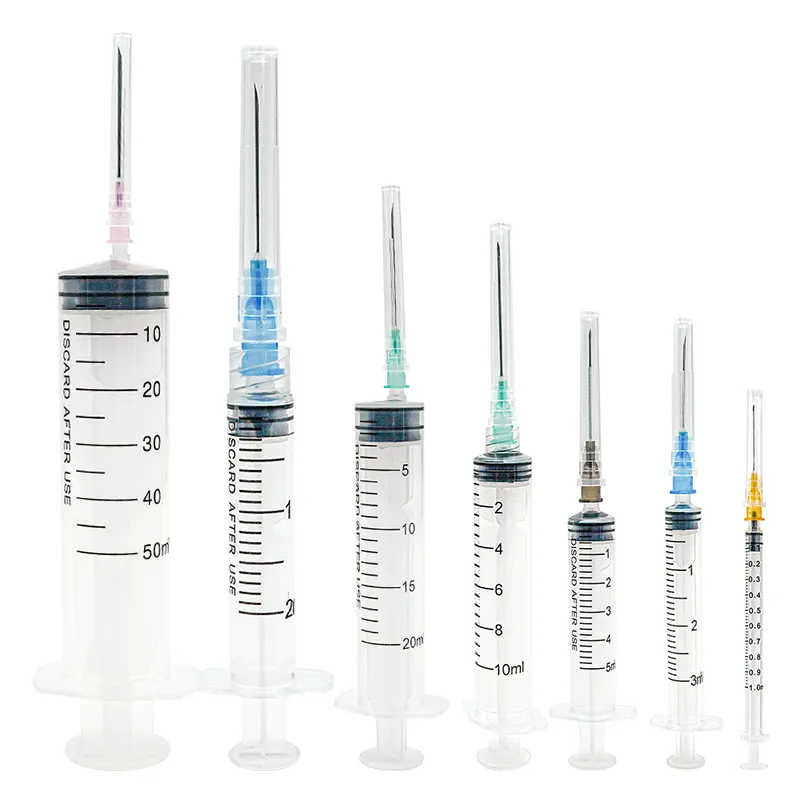
An insulin injection syringe manufacturer specializes in producing high-precision medical devices essential for diabetes management. These facilities employ state-of-the-art automation systems and clean room technology to ensure the production of sterile, accurate, and reliable insulin syringes. The manufacturing process encompasses multiple quality control checkpoints, from raw material inspection to final product testing. Modern facilities utilize advanced injection molding techniques to create precise barrel graduations and ensure consistent needle attachment. The manufacturer implements strict quality management systems compliant with ISO 13485 standards and FDA regulations. The production line includes automated assembly systems for needle mounting, barrel marking, and packaging processes. These facilities also maintain rigorous environmental controls to prevent contamination and ensure product sterility. The manufacturer's capabilities typically include various syringe sizes, from 0.3mL to 1mL, with different needle gauges to accommodate various insulin types and patient needs. Research and development teams continuously work to improve product design, focusing on features like needle sharpness, smooth plunger action, and clear markings for accurate dosing. The facility's quality assurance program includes regular testing for needle strength, attachment security, and marking permanence to ensure consistent product performance.