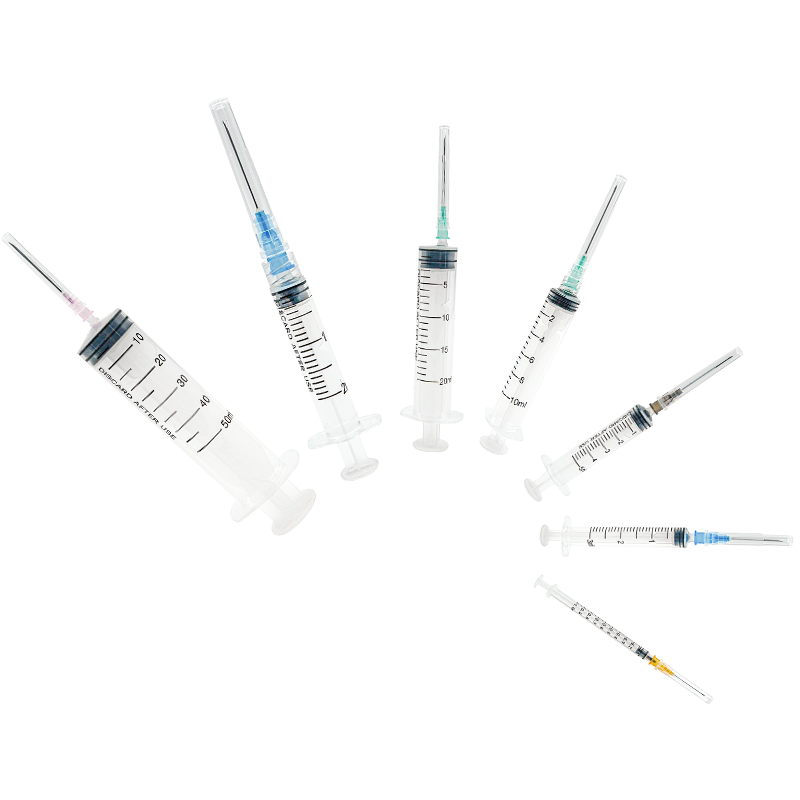
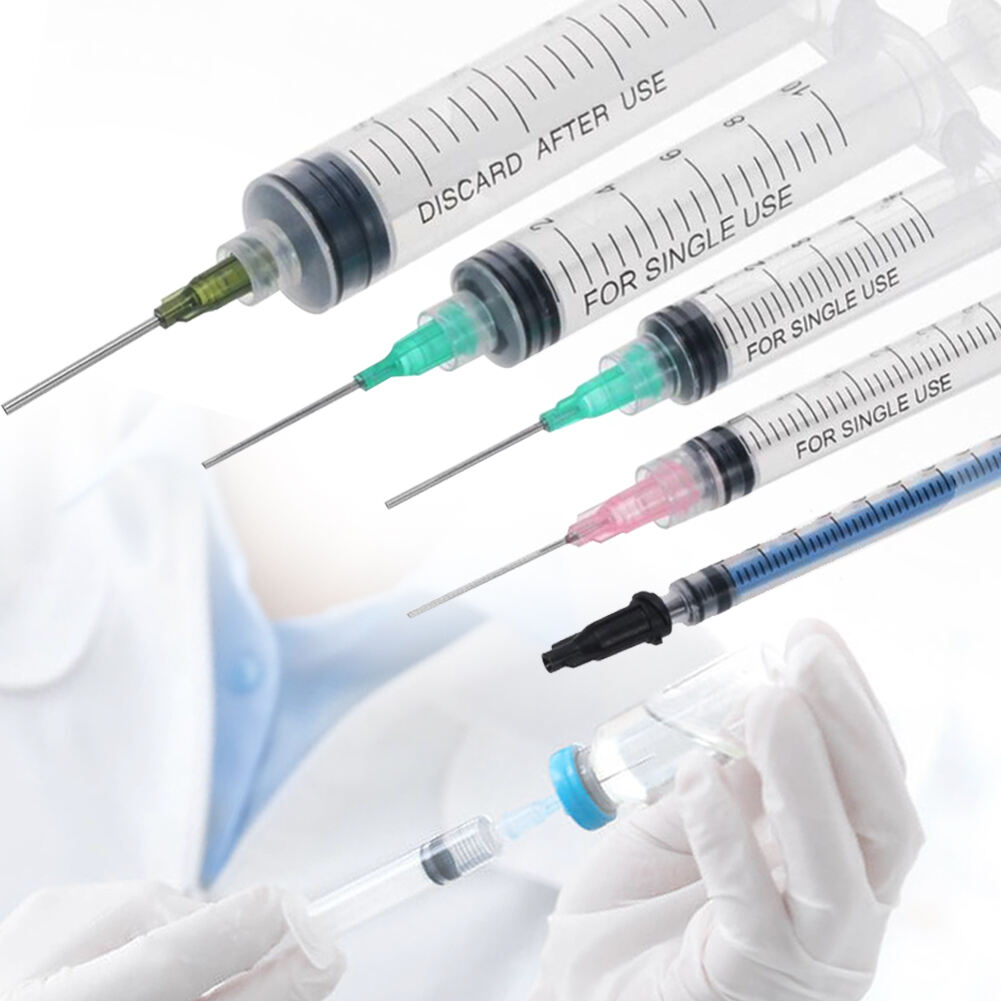
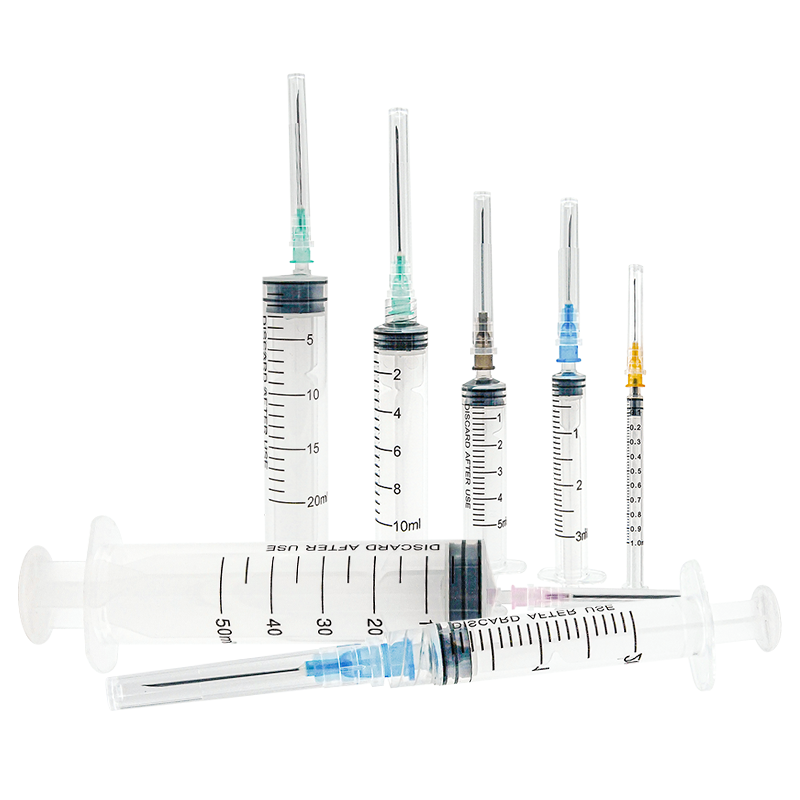
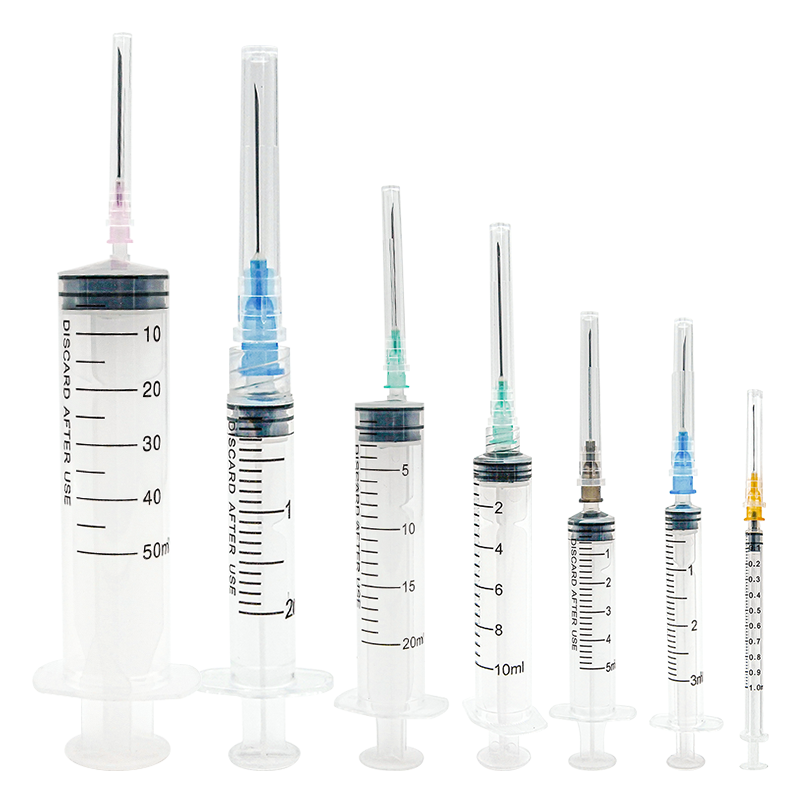
hersteller von 50 ml Einwegspritzen
Ein Hersteller von 50 ml Einwegspritzen spezialisiert sich auf die Produktion hochwertiger Medizingeräte, die für medizinische Anwendungen unerlässlich sind. Diese Hersteller verwenden fortgeschrittene Spritzgusstechnologie und automatisierte Montagelinien, um eine präzise Fertigung steriler, einweg verwendbarer Spritzen zu gewährleisten. Die Anlagen verfügen über die ISO 13485 Zertifizierung und halten sich an strenge GMP- Standards, was Produkt Sicherheit und Zuverlässigkeit garantiert. Der Fertigungsprozess umfasst mehrere Qualitätskontrollpunkte, von der Prüfung der Rohstoffe bis hin zum Testen des Endprodukts. Die Produktionslinien sind mit modernster Maschinerie ausgestattet, die Millionen von Einheiten monatlich produzieren kann, während sie eine konsistente Qualität aufrechterhält. Diese Hersteller implementieren strenge Sterilisierungsprotokolle, typischerweise mit Ethylenoxid oder Gammastrahlung, um die Sterilität der Produkte sicherzustellen. Die Spritzen sind so konzipiert, dass sie einen glatten Kolbenhub, klare Markierungen am Zylinder und sichere Luer-Lock- oder Luer-Slip-Verbindungen bieten. Sie decken verschiedene medizinische Anwendungen ab, einschließlich Flüssigkeitsverabreichung, Probenentnahme und medizinischer Forschung. Die Produktionsanlagen unterhalten außerdem Reinraum-Umgebungen, um Kontaminationen zu verhindern und die Produktintegrität während des gesamten Produktionsprozesses sicherzustellen.