20 ml disposable syringe manufacturer
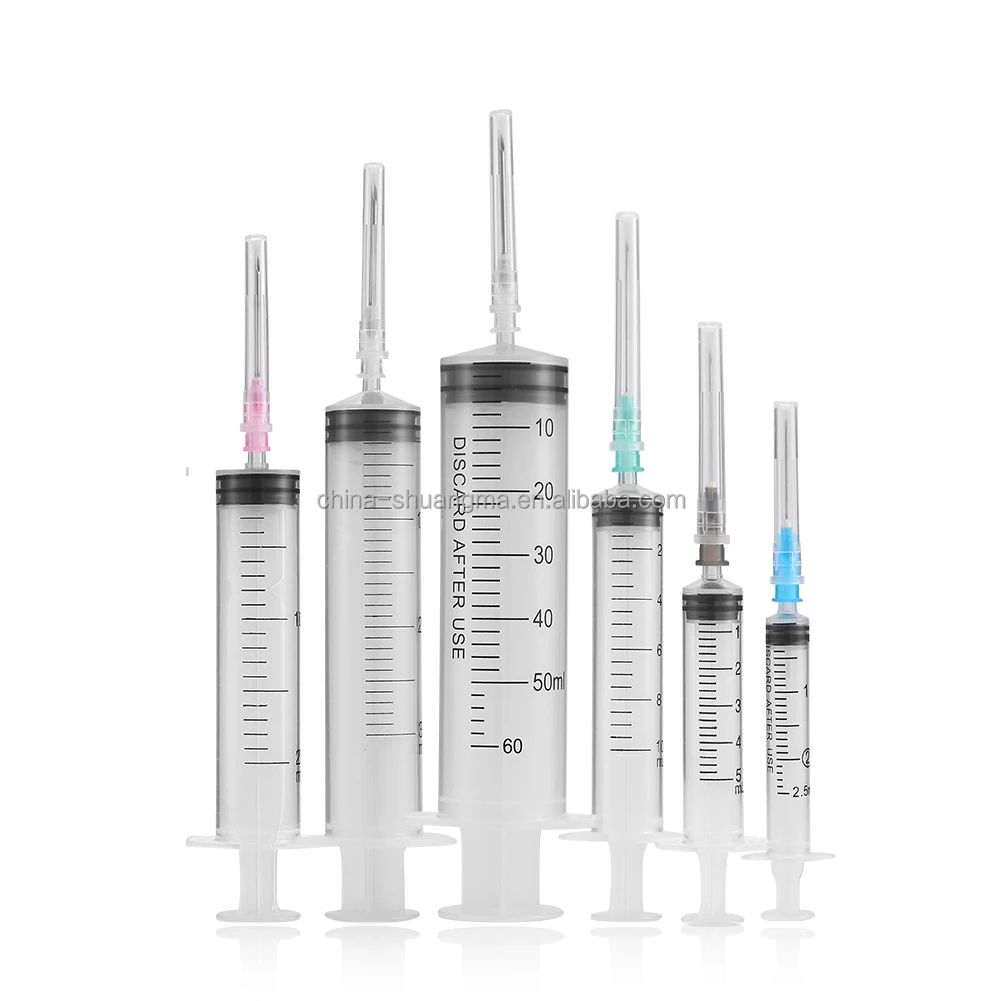
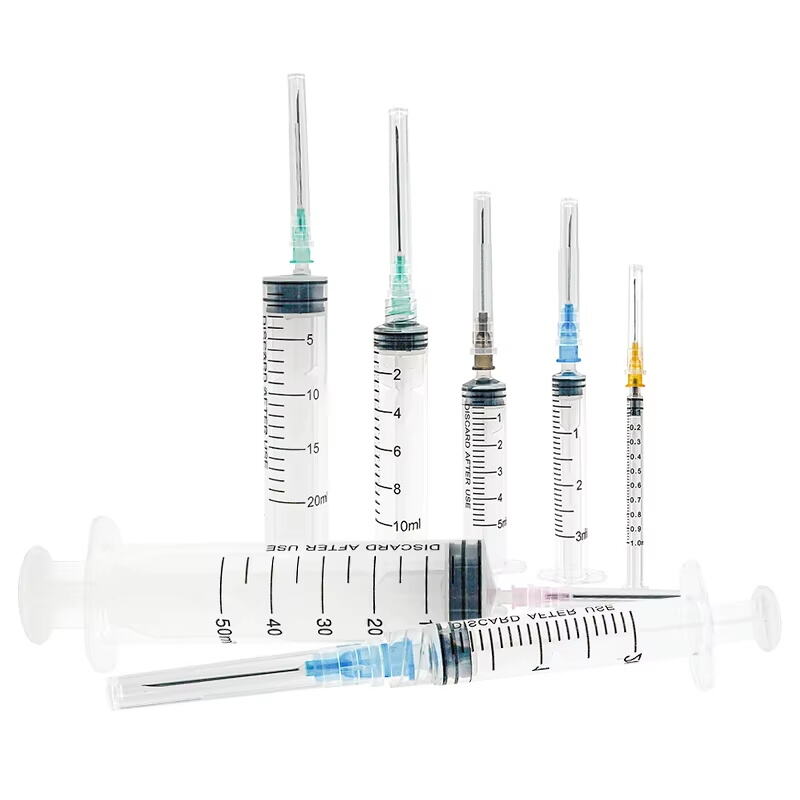
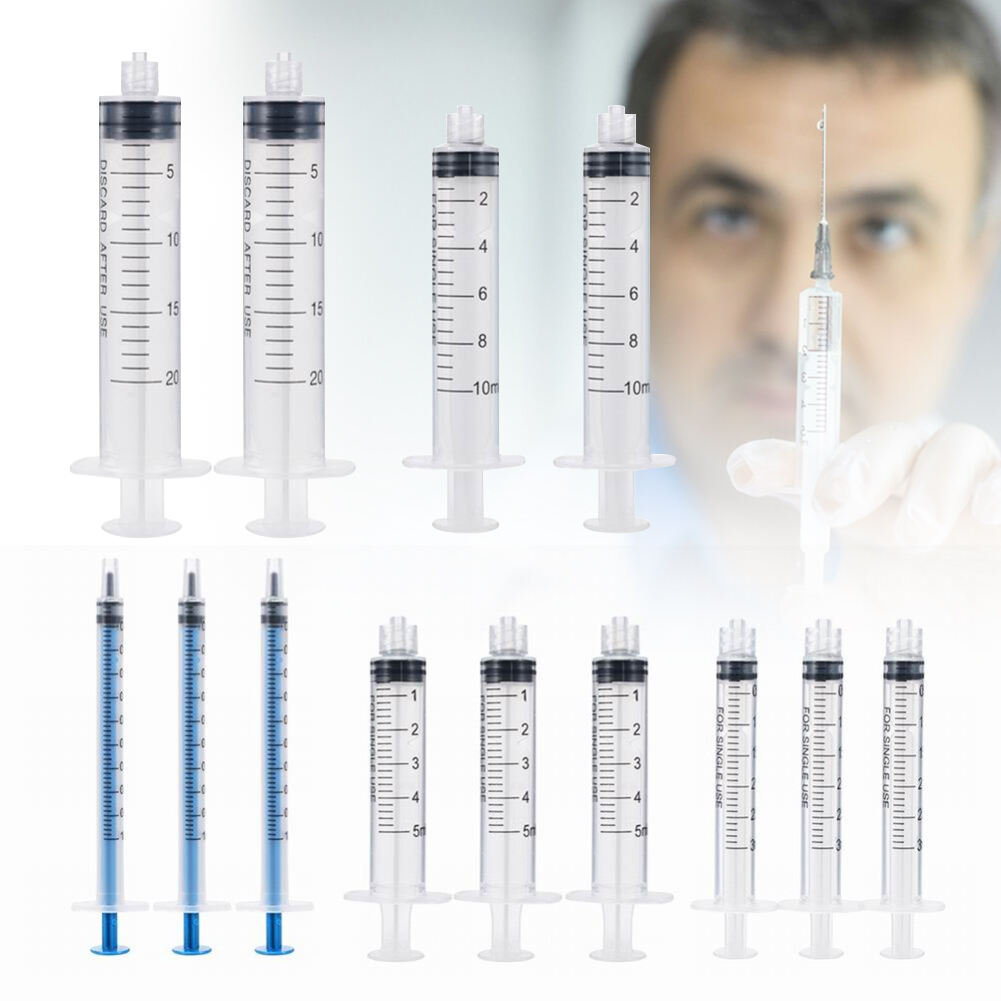
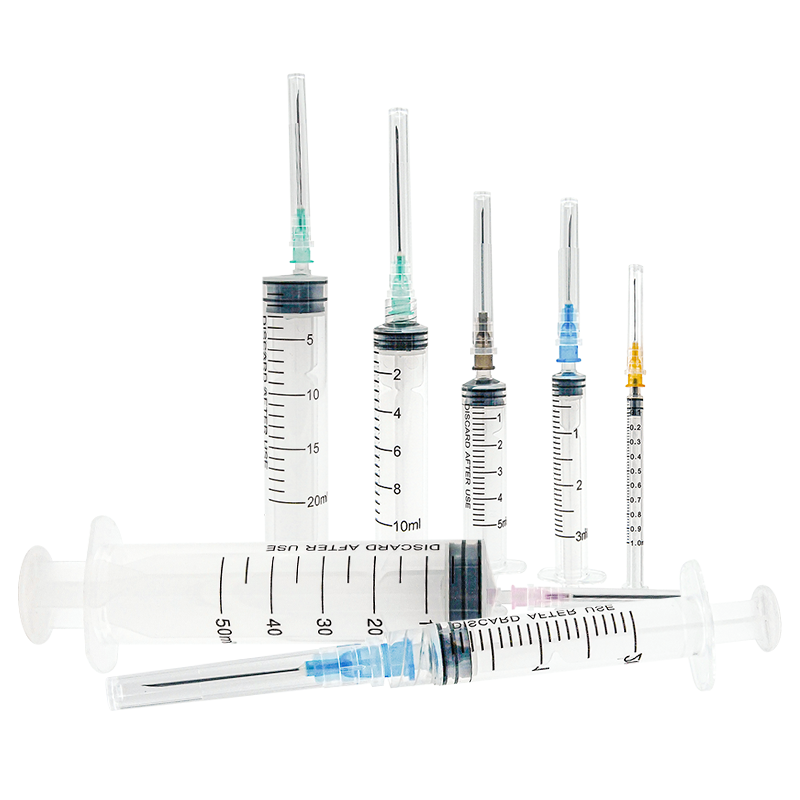
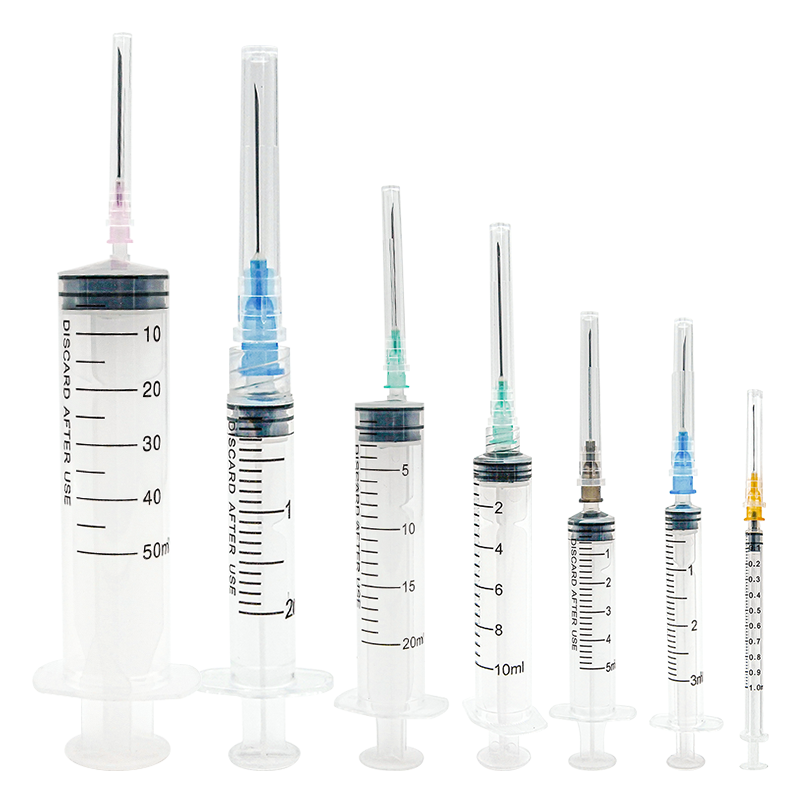
A 20 ml disposable syringe manufacturer specializes in producing high-quality medical devices essential for healthcare settings worldwide. These manufacturers employ advanced automation systems and precision engineering to create sterile, accurate, and reliable syringes that meet international quality standards. The production facilities utilize state-of-the-art clean rooms and automated assembly lines to ensure consistent quality and contamination-free products. The manufacturing process incorporates medical-grade materials, primarily polypropylene for the barrel and high-density polyethylene for the plunger, ensuring biocompatibility and chemical resistance. These facilities implement strict quality control measures, including regular testing for material integrity, dimensional accuracy, and sterilization effectiveness. The syringes feature clear graduation marks for precise measurement, smooth plunger action for controlled delivery, and secure luer lock or slip tip connections for various medical applications. Manufacturers typically maintain ISO 13485 certification and comply with FDA regulations, demonstrating their commitment to quality and safety. The production capacity often reaches millions of units monthly, serving hospitals, clinics, research facilities, and pharmaceutical companies globally.