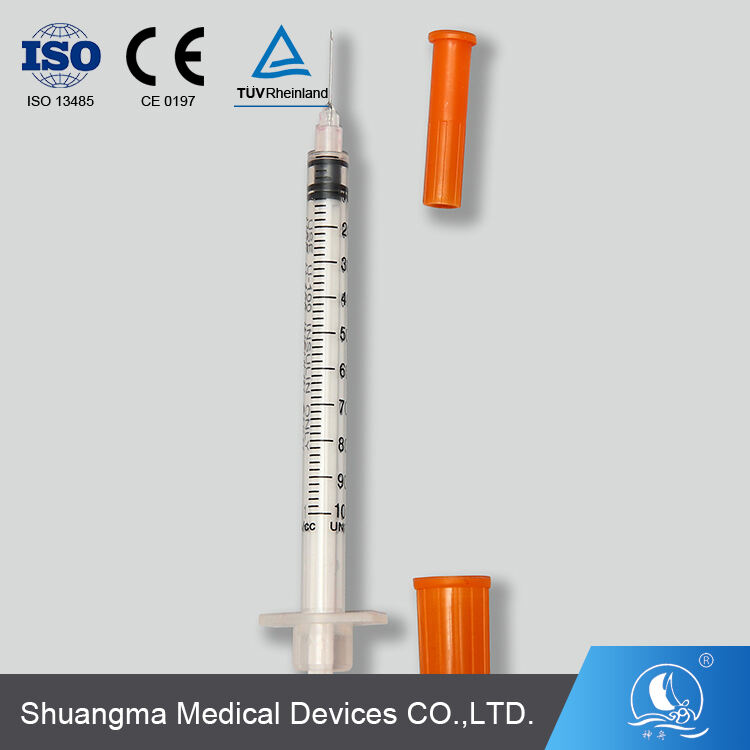
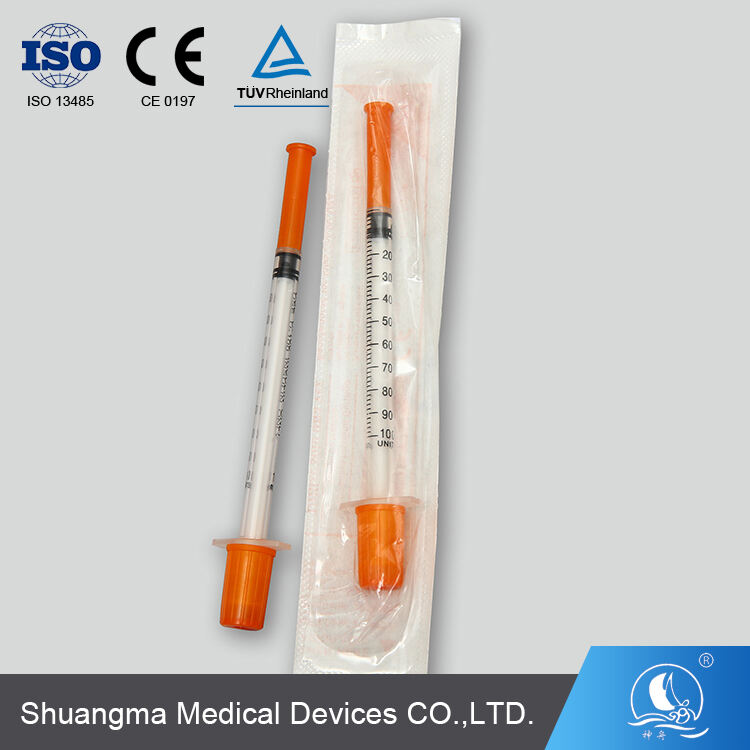
single use insulin syringes manufacturer
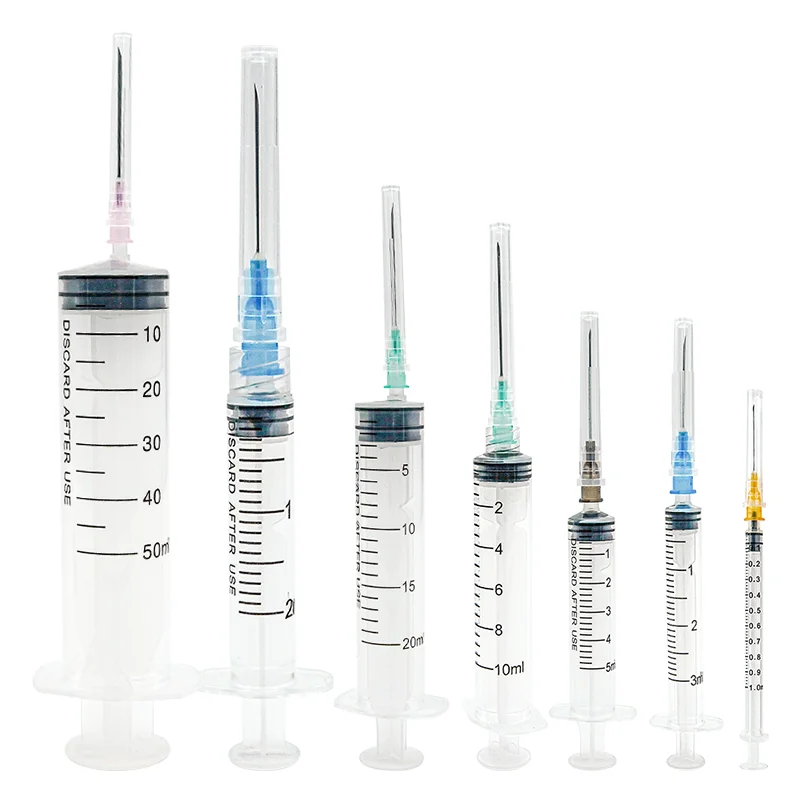
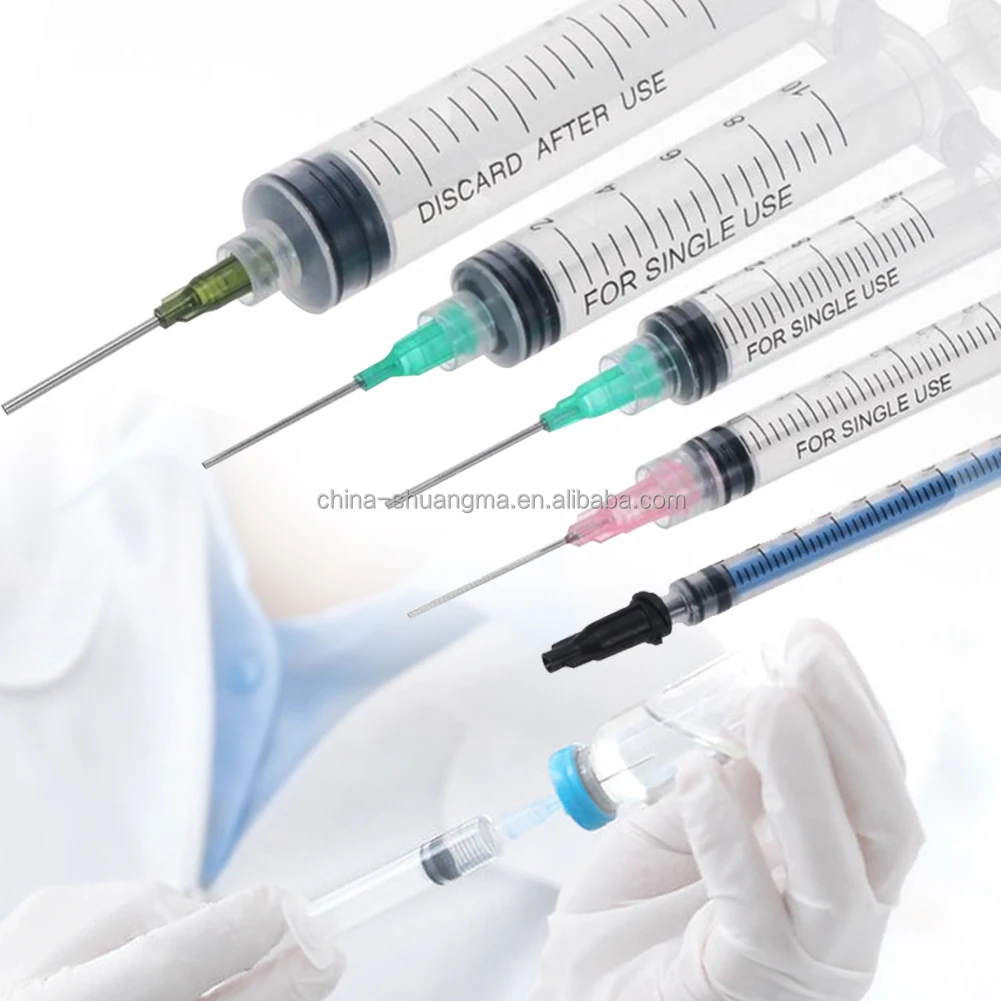
A single use insulin syringes manufacturer specializes in producing high-precision medical devices essential for diabetes management. These facilities employ advanced manufacturing processes and strict quality control measures to create sterile, disposable syringes specifically designed for insulin administration. The manufacturing process incorporates state-of-the-art automation systems, clean room technology, and precision engineering to ensure consistent product quality. These facilities utilize medical-grade materials, including specialized plastics and ultra-fine needles, to produce syringes that minimize injection pain and maximize dosing accuracy. The manufacturing process includes multiple quality checkpoints, from raw material inspection to final product testing, ensuring compliance with international medical device standards and regulations. The facilities are equipped with advanced molding technologies for producing syringe barrels with clear, precise graduation marks, enabling accurate dosing. Additionally, they implement automated needle attachment processes and sterilization systems to maintain product integrity. These manufacturers often integrate research and development departments to continuously improve product designs and manufacturing efficiency, focusing on patient comfort and safety while meeting the growing global demand for insulin delivery devices.