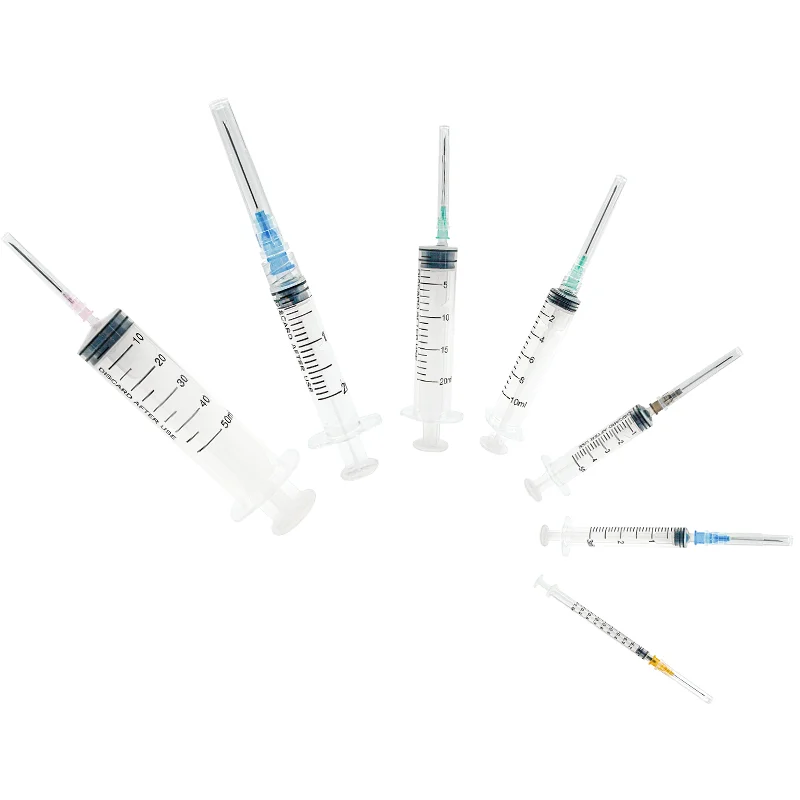
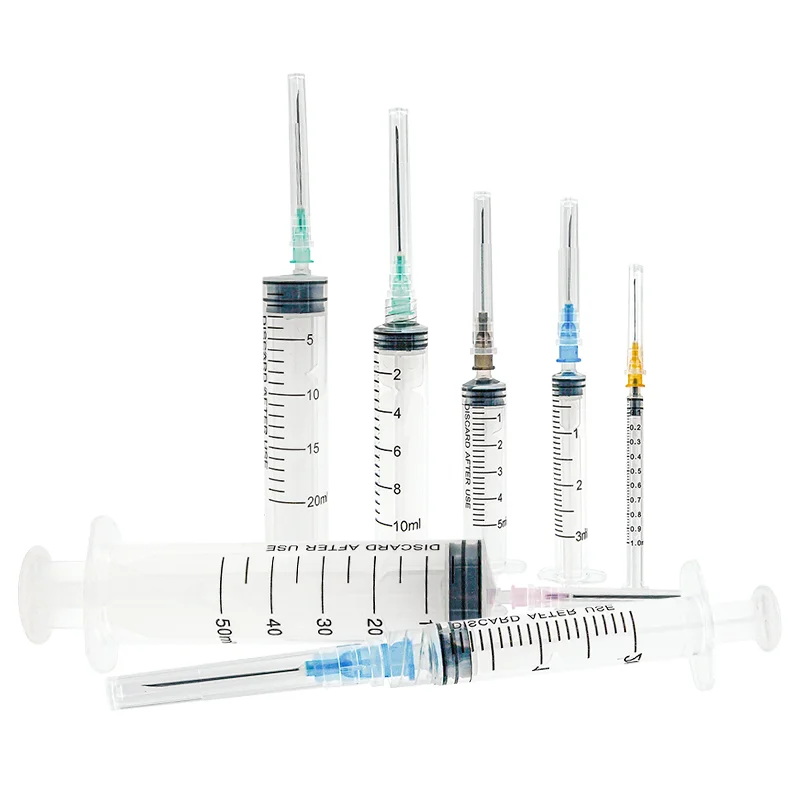
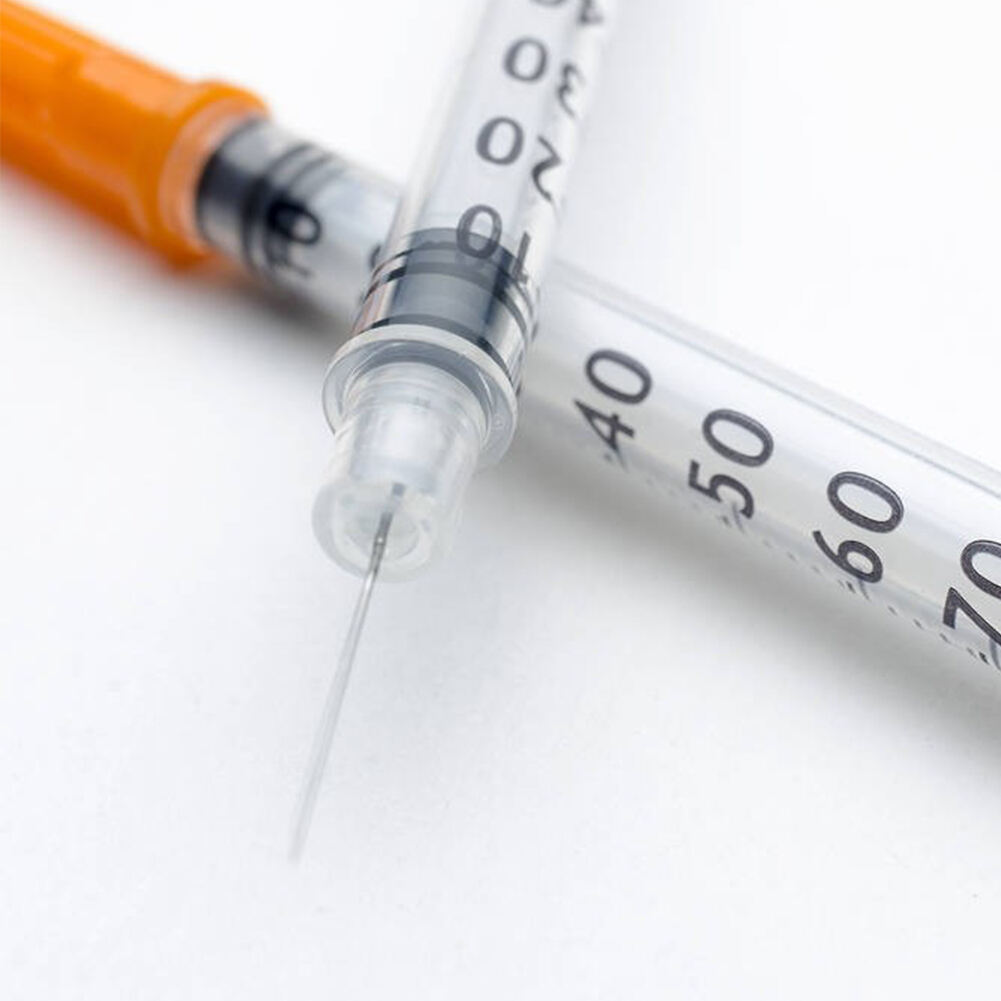
31 gauge insulin syringes manufacturer
A 31 gauge insulin syringes manufacturer specializes in producing high-precision medical devices essential for diabetes management and other medical applications. These manufacturers employ state-of-the-art production facilities equipped with advanced automation systems to ensure consistent quality and precision in every syringe produced. The 31 gauge needle, known for its ultra-thin diameter, represents the pinnacle of manufacturing excellence in medical device production. The manufacturing process incorporates stringent quality control measures, including clean room facilities and automated inspection systems, to maintain the highest standards of sterility and accuracy. These facilities utilize advanced materials science to develop specialized needle coatings that enhance comfort during injection while maintaining structural integrity. The manufacturing process also includes sophisticated testing protocols to verify needle sharpness, flow rates, and compatibility with various insulin formulations. Modern 31 gauge insulin syringe manufacturers integrate sustainable practices and environmentally conscious production methods while maintaining compliance with international medical device regulations and standards. The facilities typically feature dedicated research and development departments focused on continuous improvement in needle design, materials, and production efficiency.