disposable biopsy forceps manufacturer
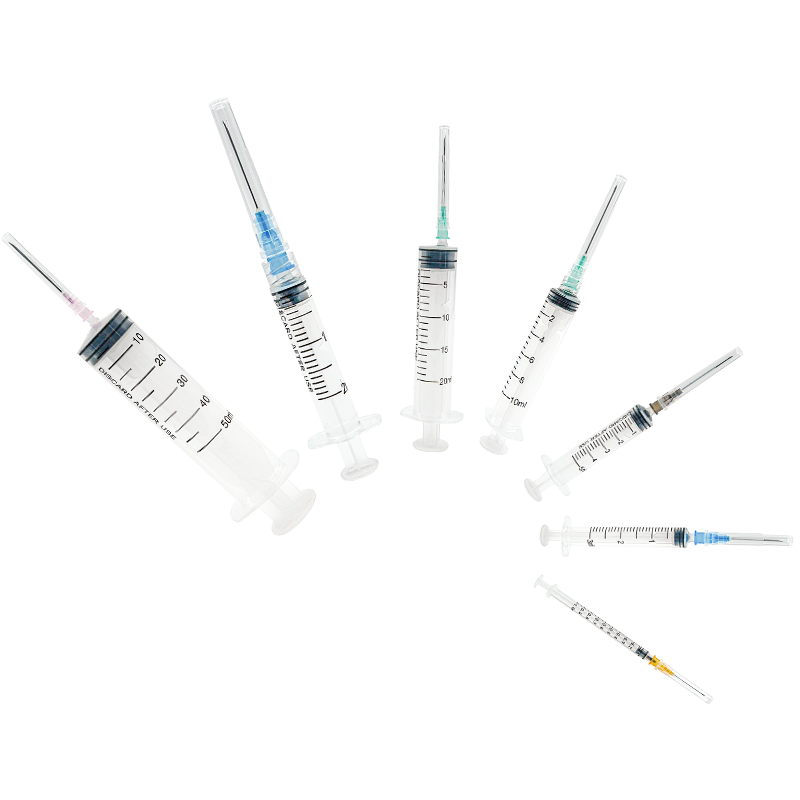
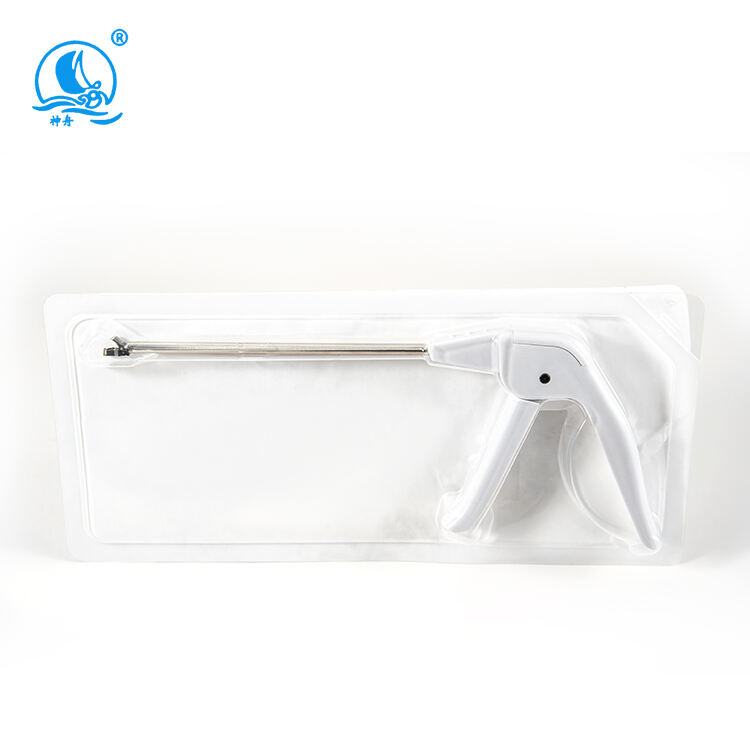
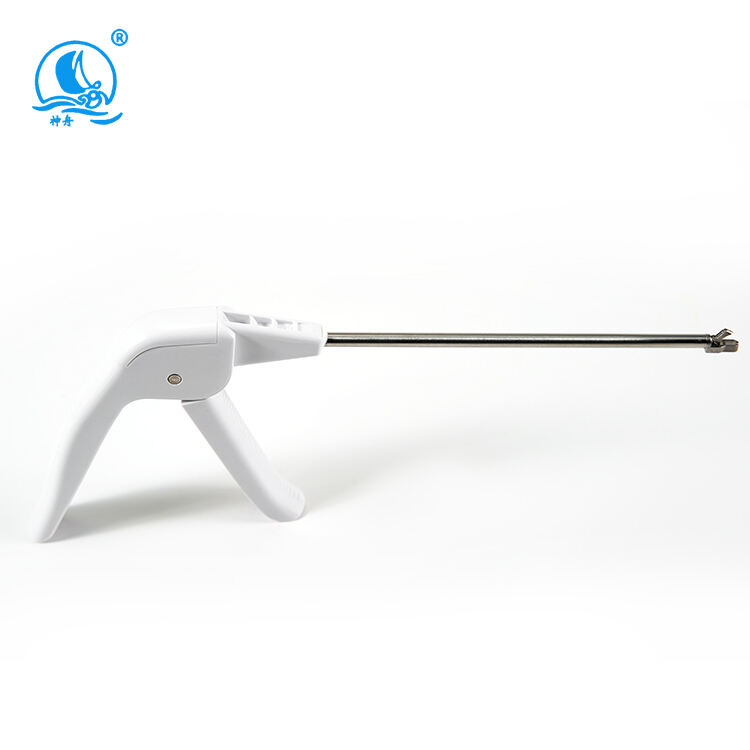
A disposable biopsy forceps manufacturer specializes in producing high-quality, single-use medical instruments designed for tissue sampling procedures. These manufacturers employ advanced manufacturing processes and state-of-the-art technology to create precision instruments that meet stringent medical standards. Their facilities are equipped with clean rooms and automated production lines that ensure consistent quality and sterility. The manufacturing process incorporates medical-grade materials, including surgical-quality stainless steel for the jaws and flexible, durable polymers for the shaft. These manufacturers implement rigorous quality control measures throughout production, from raw material testing to final product inspection. Their product range typically includes various forceps designs optimized for different biopsy procedures, such as endoscopic, bronchoscopic, and laparoscopic applications. The manufacturing facilities maintain ISO certification and comply with FDA regulations, ensuring their products meet international safety and quality standards. They also invest in research and development to improve product designs, enhance functionality, and incorporate user feedback into new product iterations.