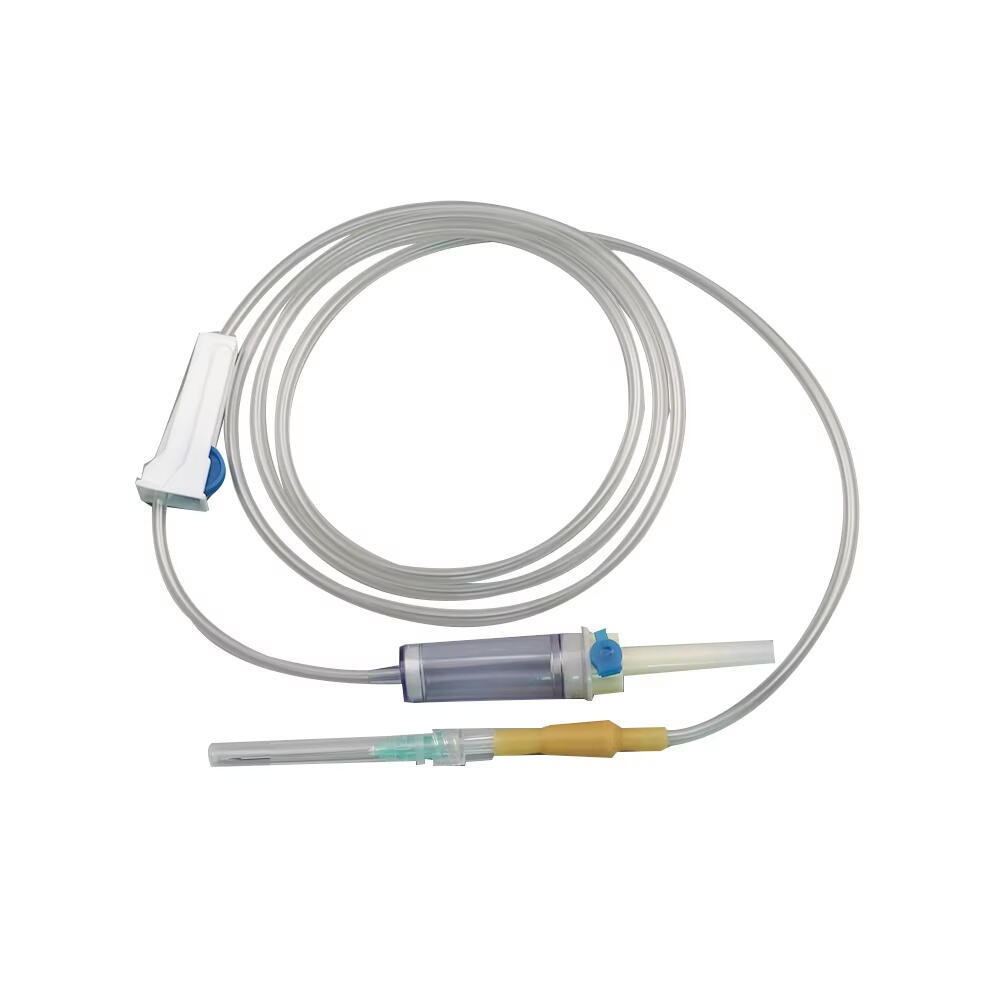
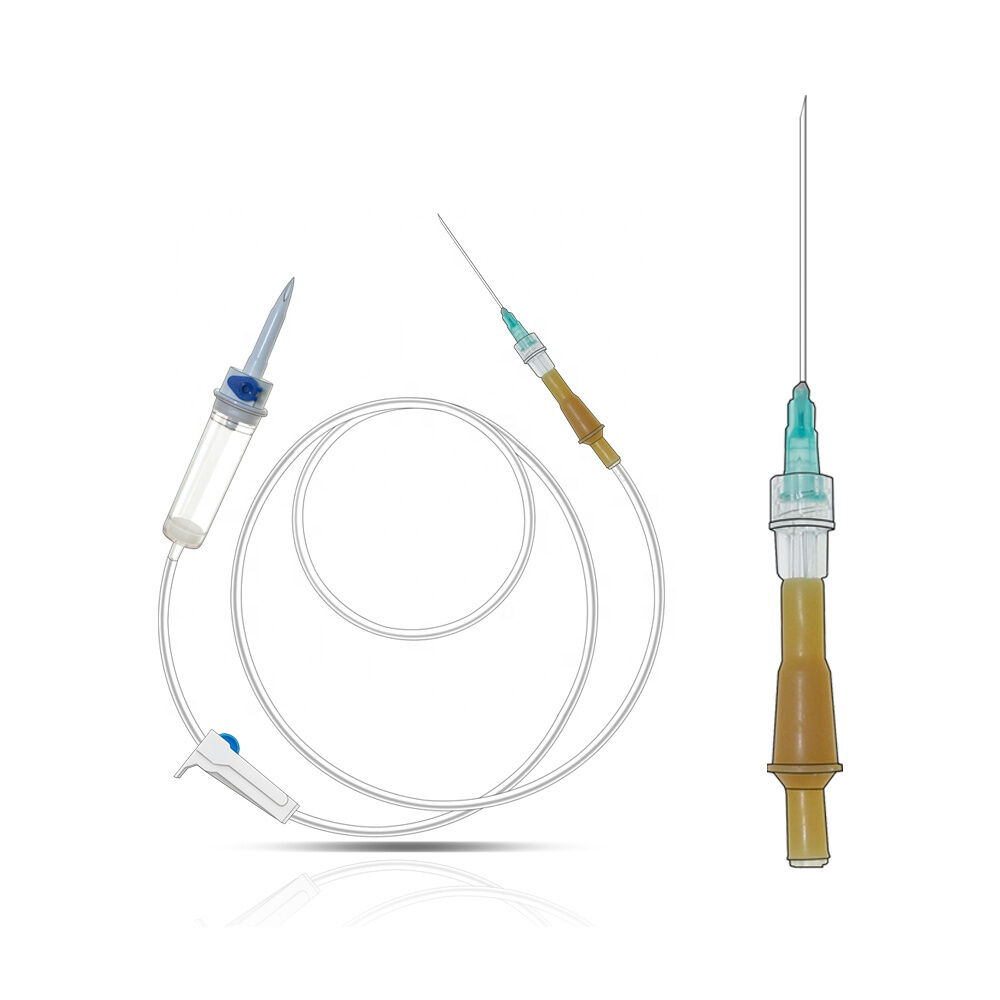
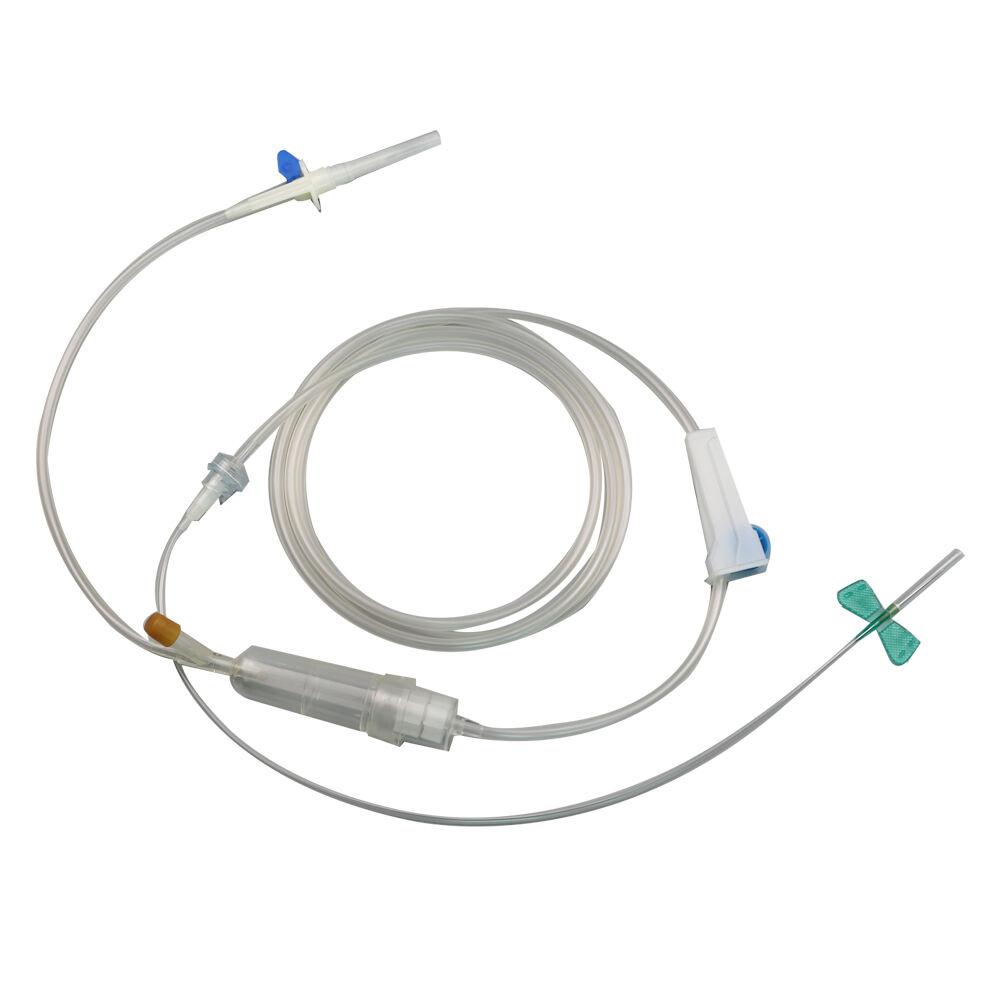
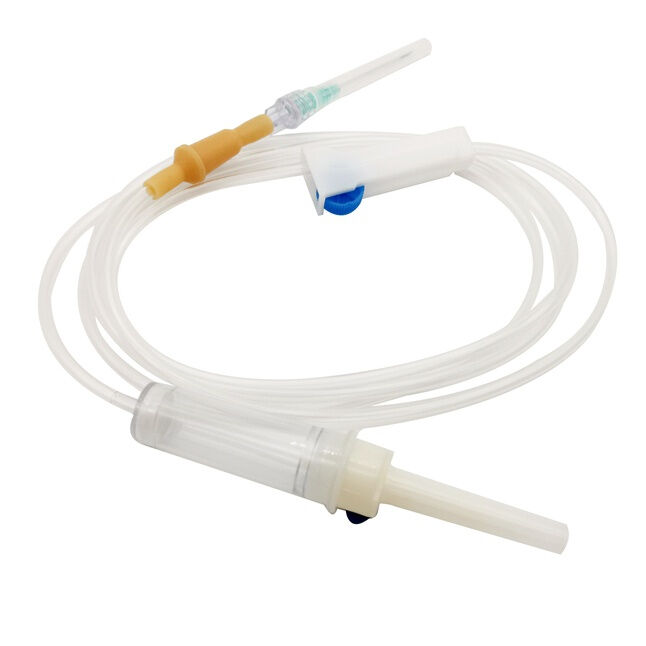
burette iv set manufacturer
A burette IV set manufacturer specializes in producing precision medical devices essential for accurate fluid administration in healthcare settings. These manufacturers employ state of the art technology and rigorous quality control processes to create reliable burette systems that ensure precise measurement and delivery of intravenous medications and fluids. The manufacturing facilities feature advanced clean room environments, automated assembly lines, and sophisticated testing equipment to maintain the highest standards of product quality and safety. These manufacturers typically offer a comprehensive range of burette IV sets, including micro burettes for pediatric use, macro burettes for adult patients, and specialized sets for specific medical applications. Each product undergoes extensive testing for flow accuracy, material compatibility, and sterility. The manufacturing process incorporates medical grade materials, precise calibration systems, and innovative design features such as clear graduated markings, smooth flow control mechanisms, and secure connection ports. Modern burette IV set manufacturers also focus on incorporating user friendly features like anti reflux valves, needle free ports, and ergonomic designs to enhance healthcare provider efficiency and patient safety. They maintain strict compliance with international medical device standards and regulations, ensuring their products meet or exceed industry requirements for safety and performance.