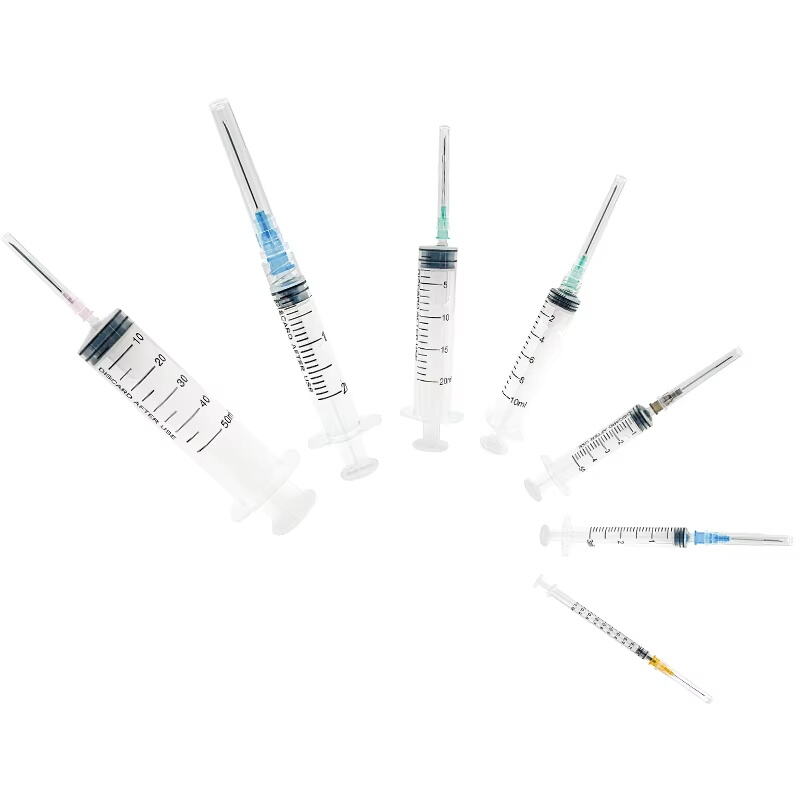
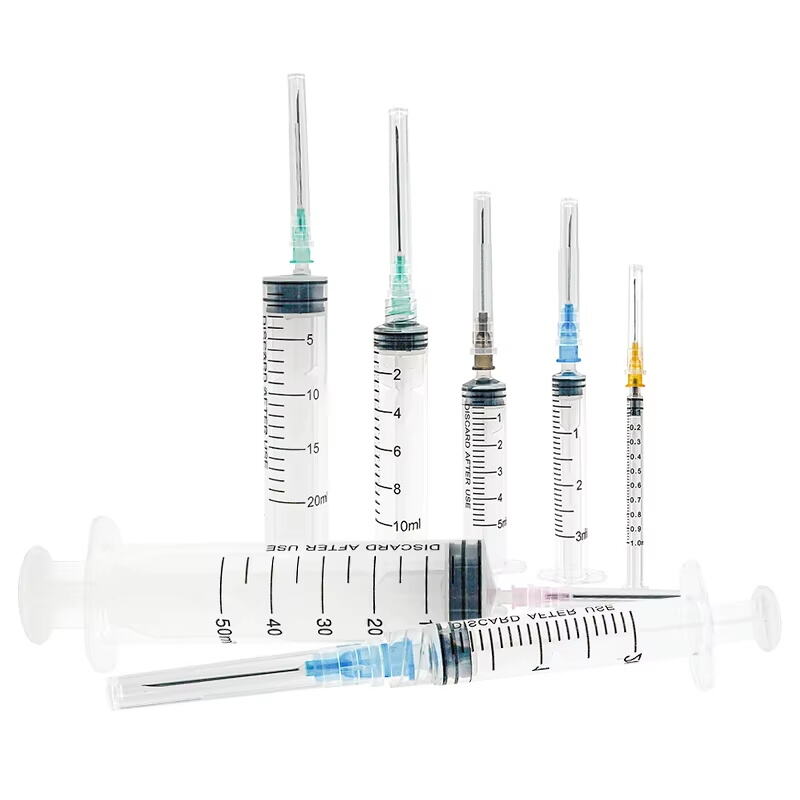
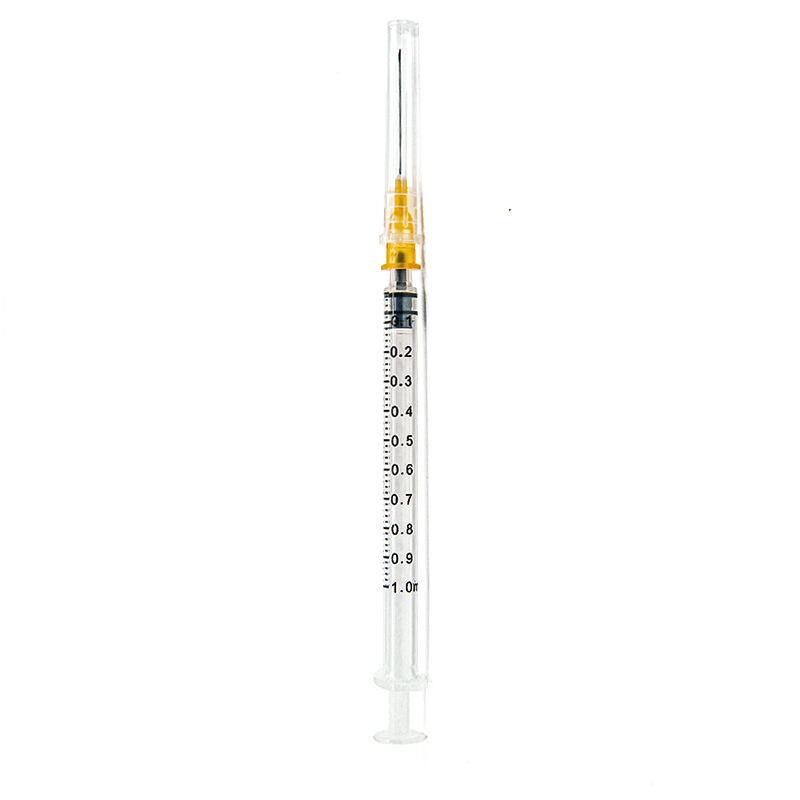
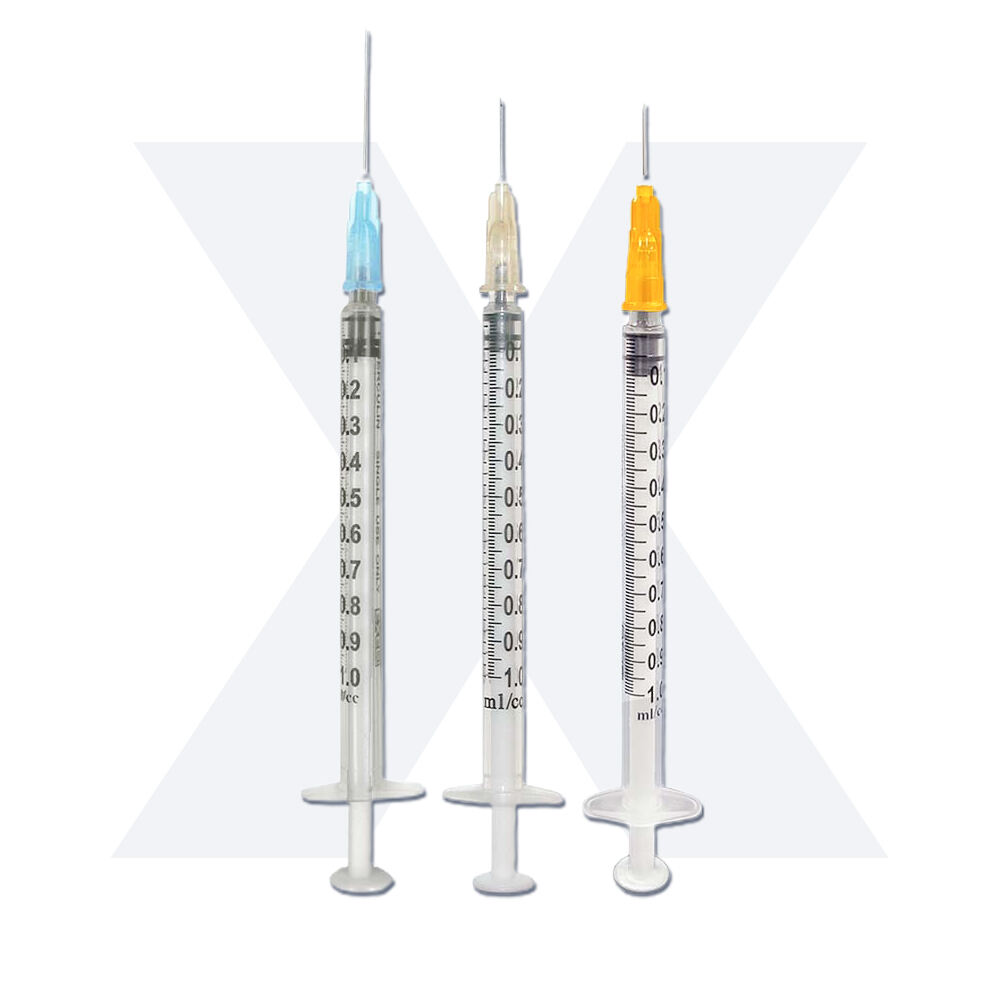
1 ml syringe without needle manufacturer
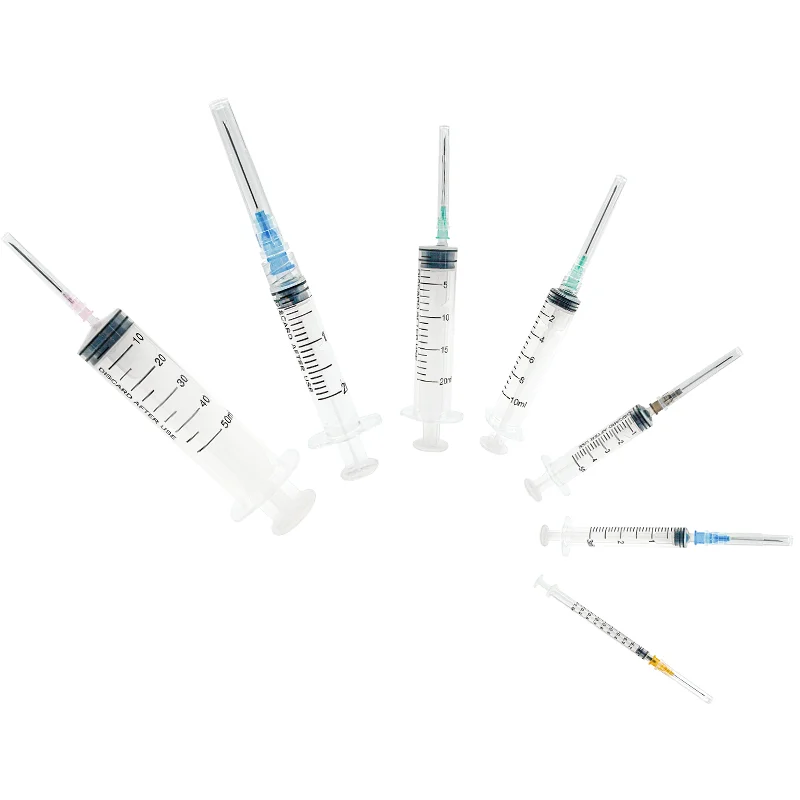
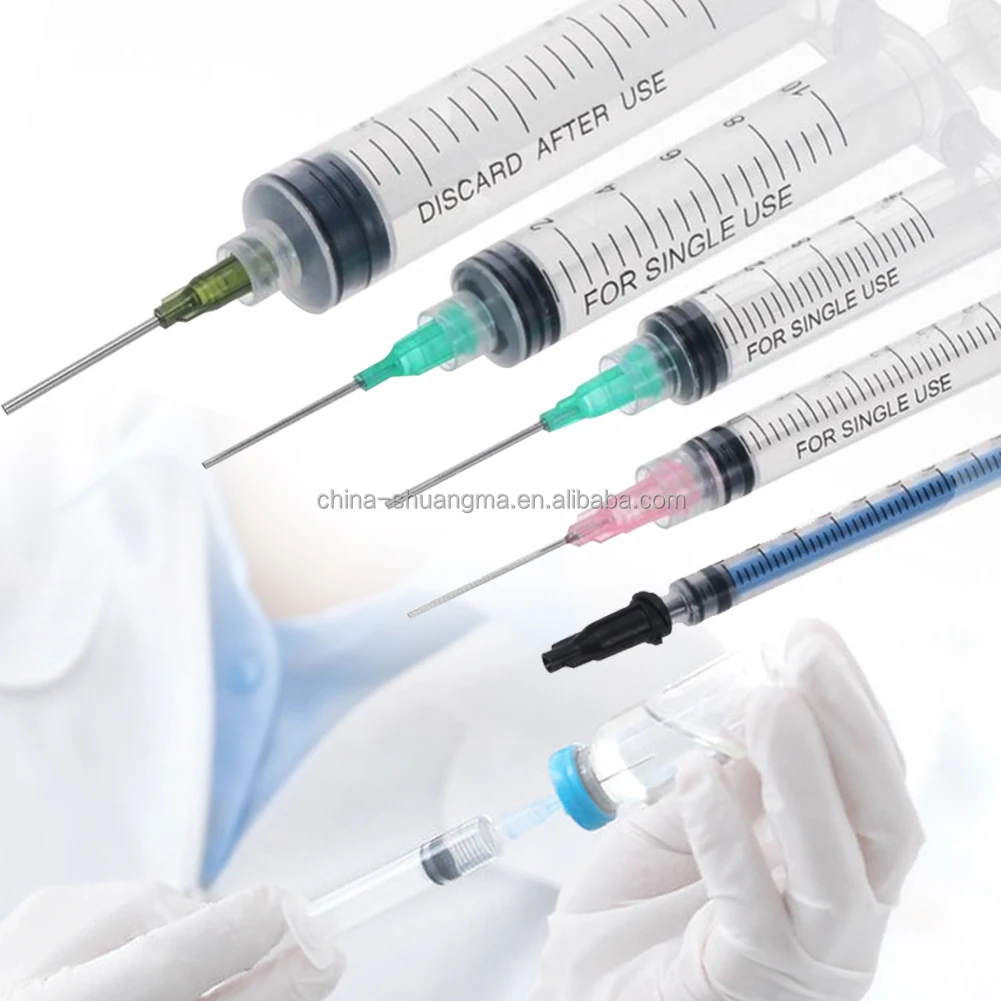
A 1 ml syringe without needle manufacturer specializes in producing precision medical devices essential for accurate liquid measurement and delivery in healthcare settings. These manufacturers employ state-of-the-art production facilities equipped with advanced injection molding technology and automated assembly lines to ensure consistent quality and sterility. The manufacturing process adheres to strict ISO 13485 medical device standards and FDA regulations, incorporating medical-grade materials that meet USP Class VI requirements. The production line features multiple quality control checkpoints, including dimensional verification, material testing, and sterility validation. These syringes are designed with clear barrel markings for precise measurements, smooth plunger action for controlled delivery, and universal Luer slip or Luer lock connections for versatile needle attachment options. The manufacturing facility maintains cleanroom conditions to prevent contamination and ensures each batch undergoes rigorous testing for particle count, bacterial endotoxins, and mechanical functionality. These syringes find applications in various medical procedures, including insulin administration, pediatric medication delivery, and precise laboratory measurements.