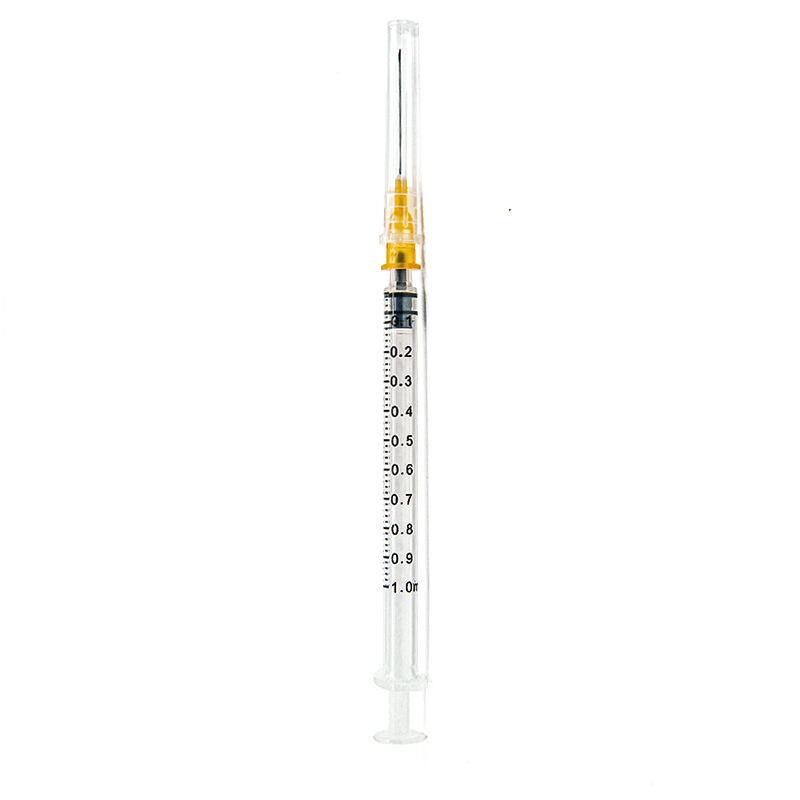
1ml sterile syringe manufacturer
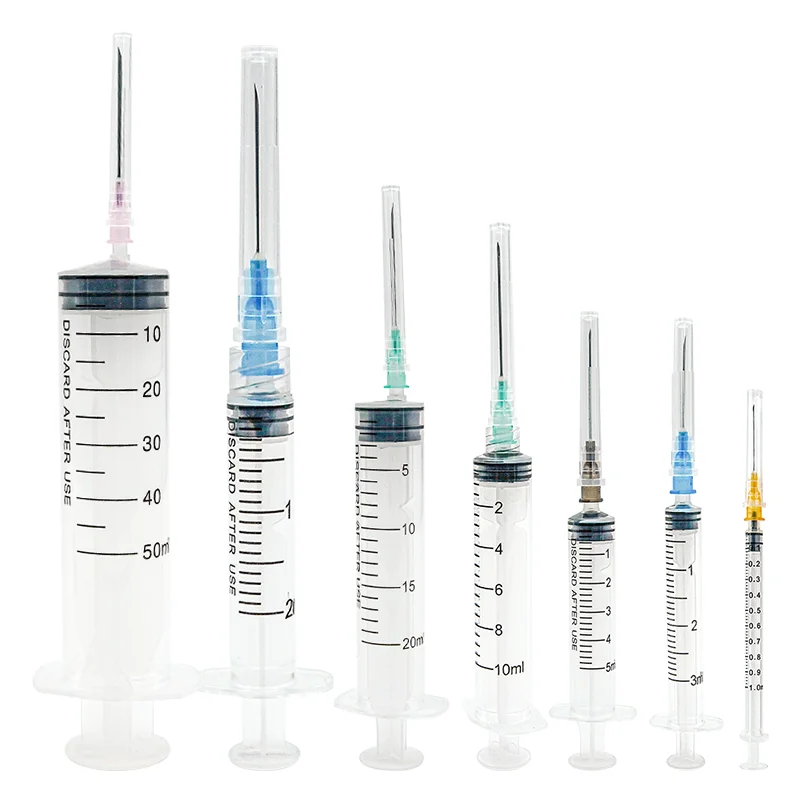
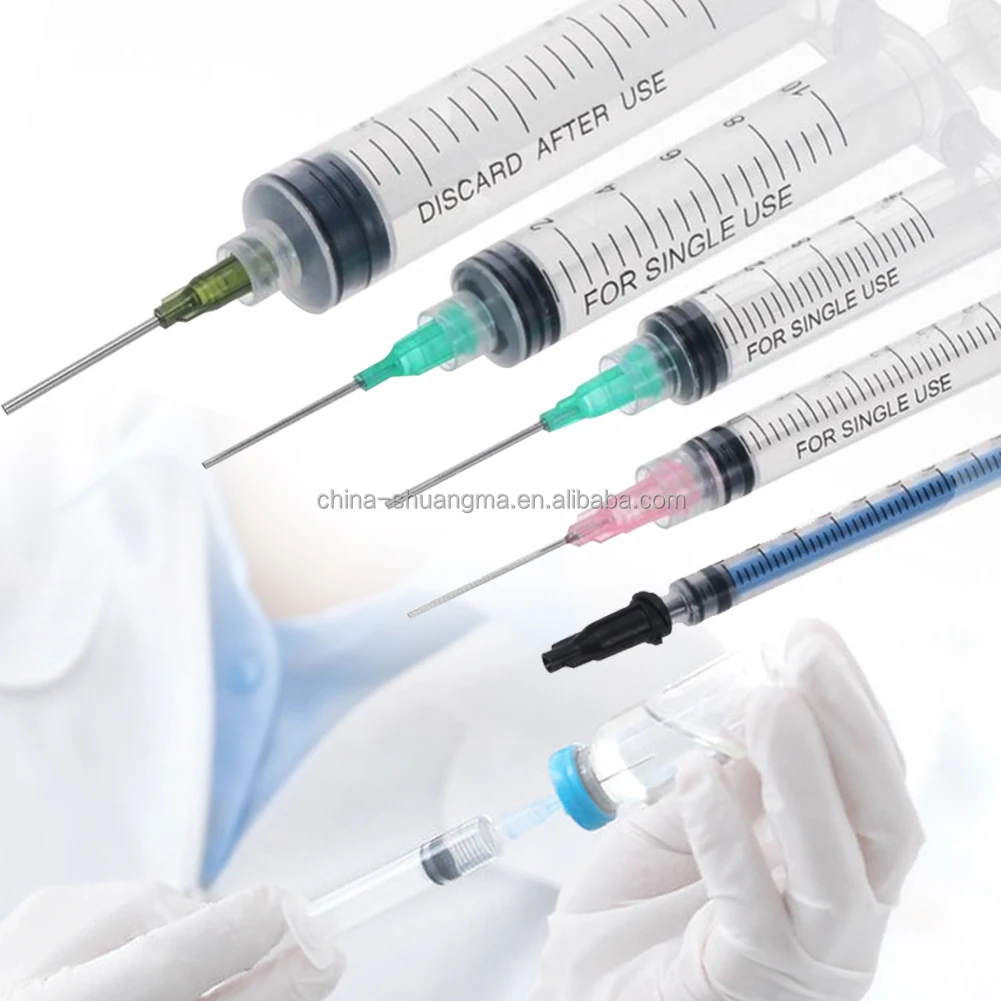
The 1ml sterile syringe manufacturer stands at the forefront of medical device production, specializing in the creation of high-precision injection tools essential for healthcare applications. Operating with state-of-the-art automated production lines, the manufacturer implements rigorous quality control measures throughout the manufacturing process. The facility maintains ISO 13485 certification and adheres to GMP standards, ensuring consistent production of sterile, medical-grade syringes. Each syringe undergoes thorough sterilization using industry-leading EtO or gamma radiation methods, guaranteeing complete sterility for safe medical use. The manufacturing process incorporates advanced polymer technology for the barrel and plunger components, ensuring smooth operation and accurate dosage delivery. The facility's production capabilities include various needle options, from standard to safety-engineered designs, meeting diverse medical requirements. With a commitment to sustainability, the manufacturer utilizes eco-friendly materials and implements waste reduction strategies in their production processes. The facility's research and development team continuously works on improving design elements, such as reducing dead space and enhancing user safety features. Quality assurance protocols include automated visual inspection systems and regular batch testing to maintain the highest standards of product reliability.