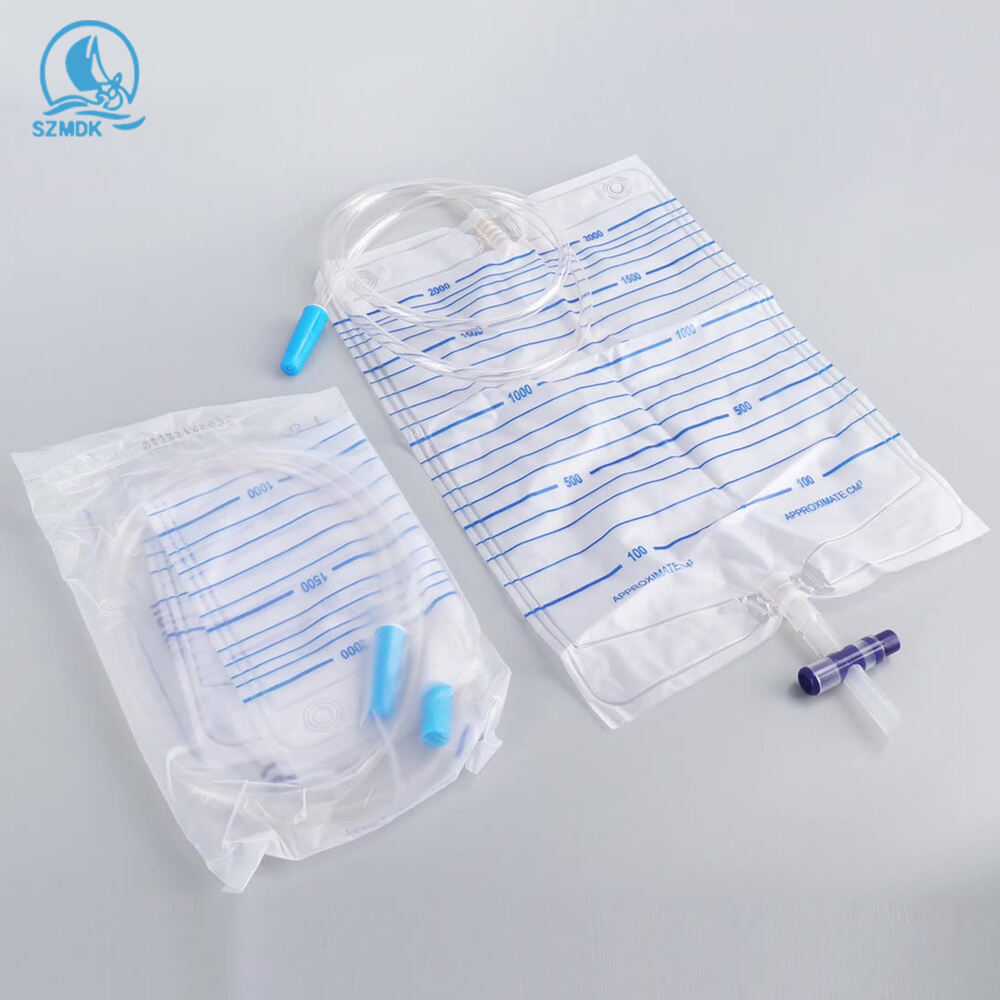
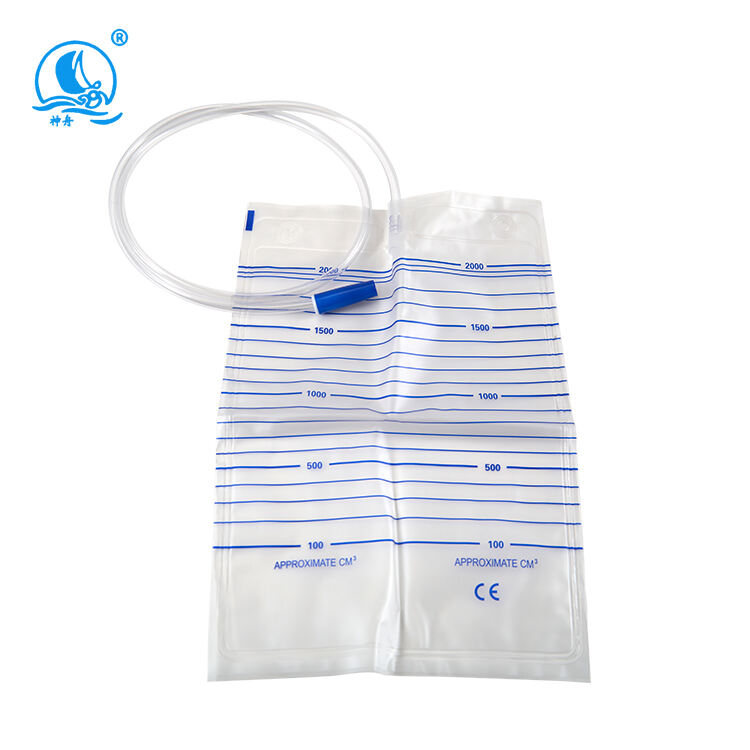
urine bag 2000ml manufacturer
As a leading urine bag 2000ml manufacturer, we specialize in producing high-capacity medical collection devices that meet stringent healthcare standards. Our manufacturing facility employs state-of-the-art production lines equipped with advanced automation systems to ensure consistent quality and precision in every product. The facility operates under strict quality control measures, implementing ISO 13485 medical device manufacturing standards throughout the production process. Our urine bags feature medical-grade PVC material, guaranteed leak-proof sealing technology, and precise volumetric measurements up to 2000ml capacity. The manufacturing process incorporates multiple quality checkpoints, from raw material testing to finished product inspection, ensuring each bag meets medical safety requirements. We utilize advanced welding techniques for secure seams and implement anti-reflux mechanisms to prevent backflow. The facility's production capability exceeds 1 million units monthly, supporting global healthcare providers with reliable medical supplies. Our research and development team continuously works on improving design elements, such as comfort-enhanced tubing and secure fastening systems, while maintaining cost-effectiveness in production.