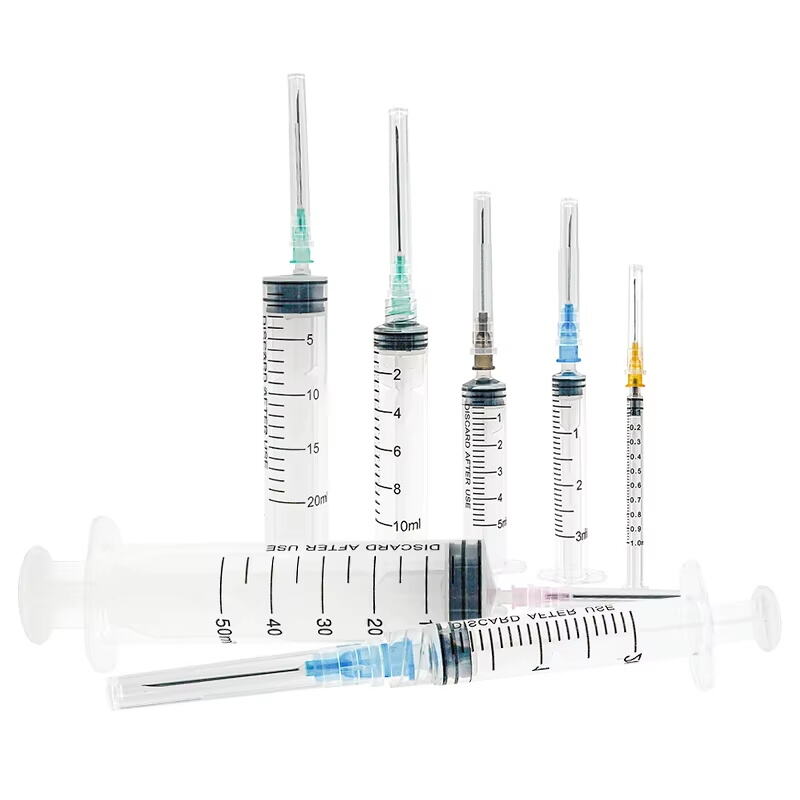
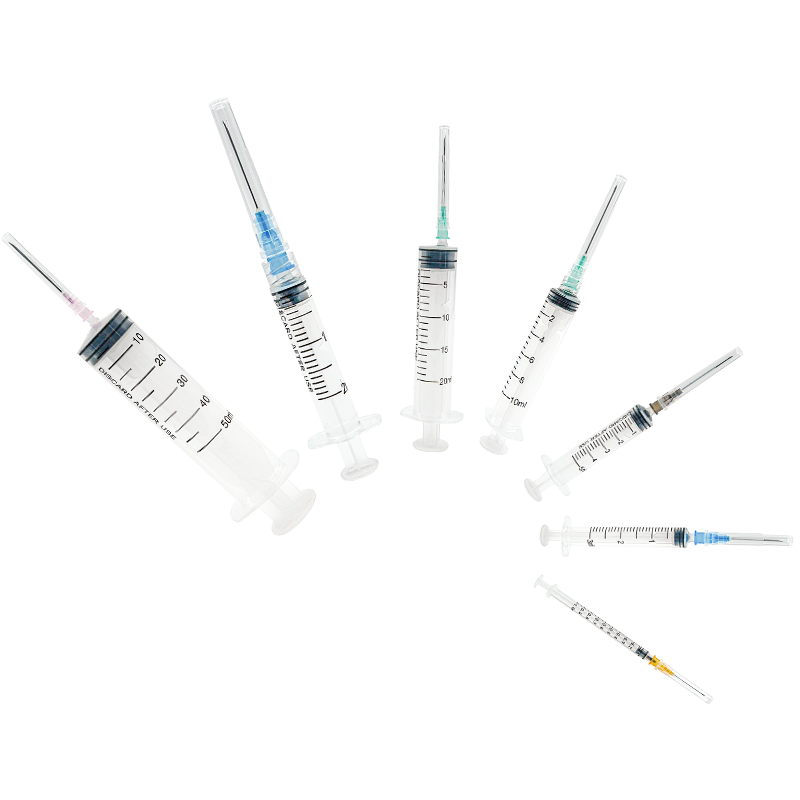
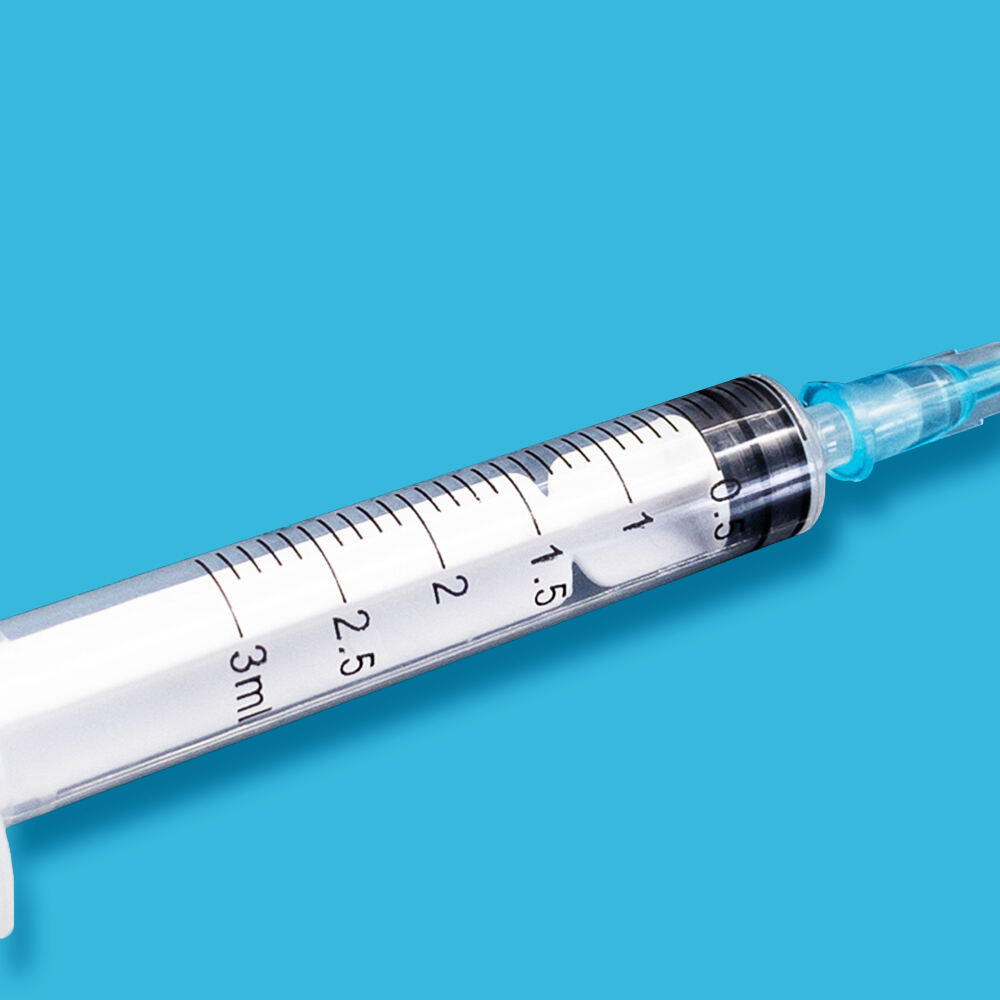
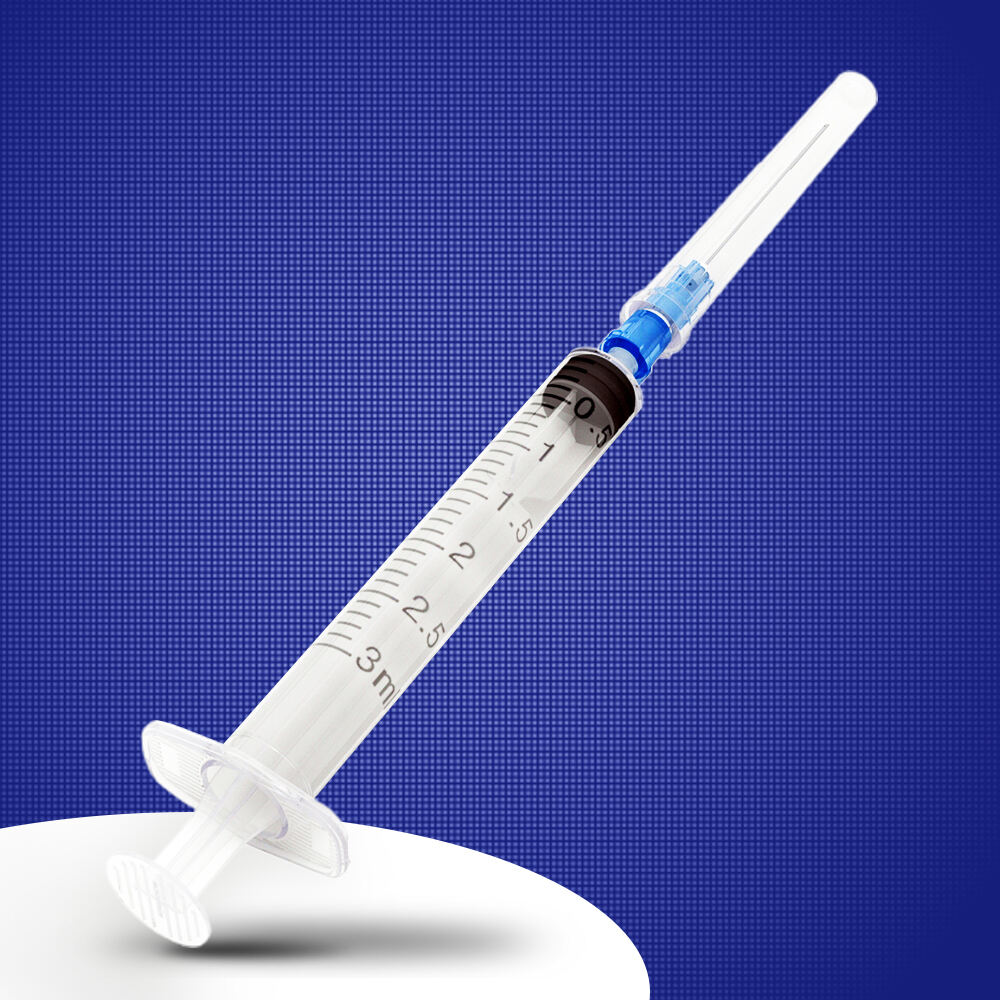
3 ml syringe no needle manufacturer
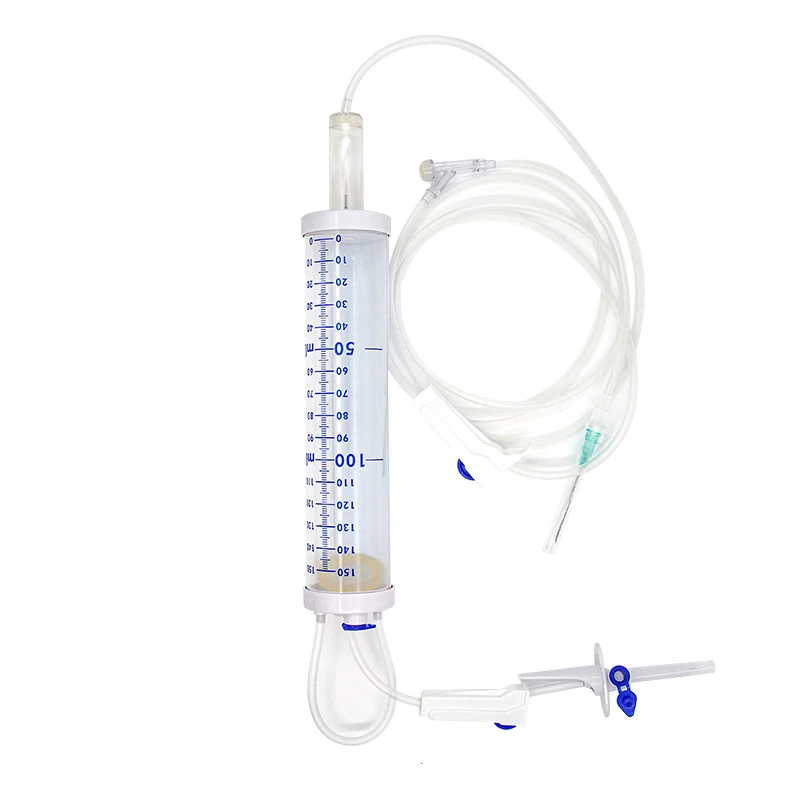
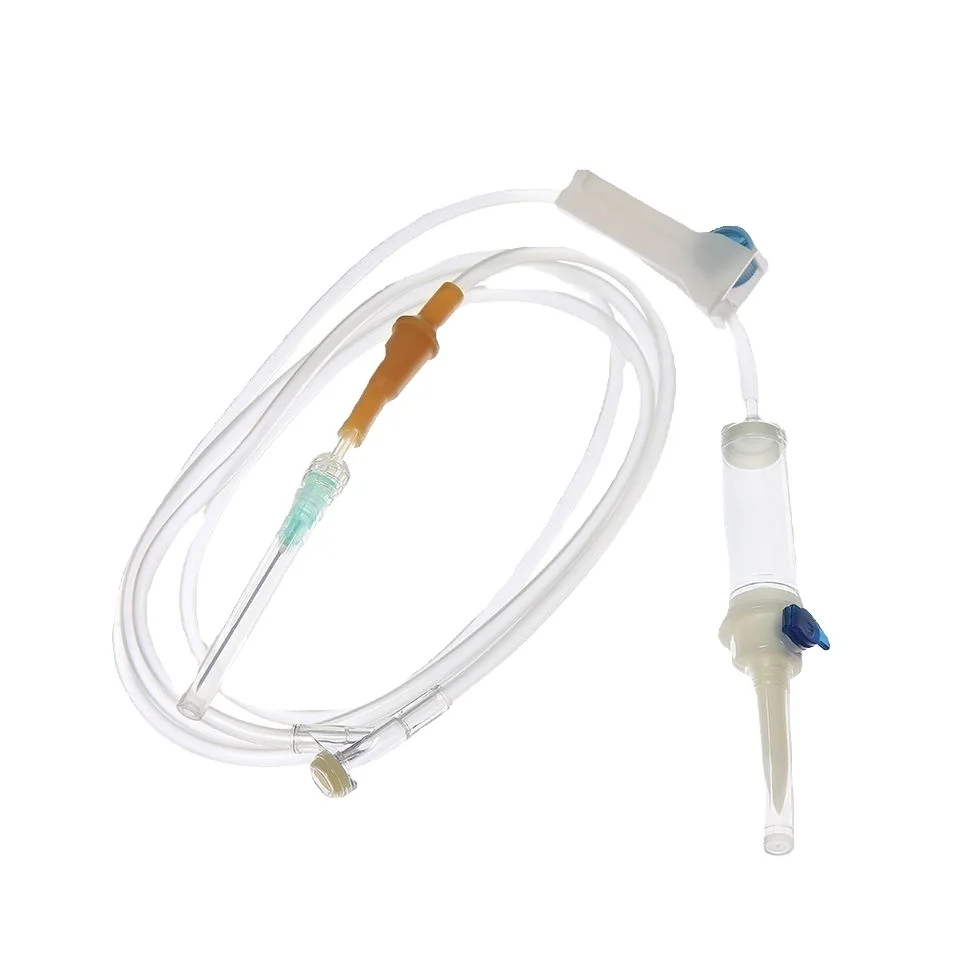
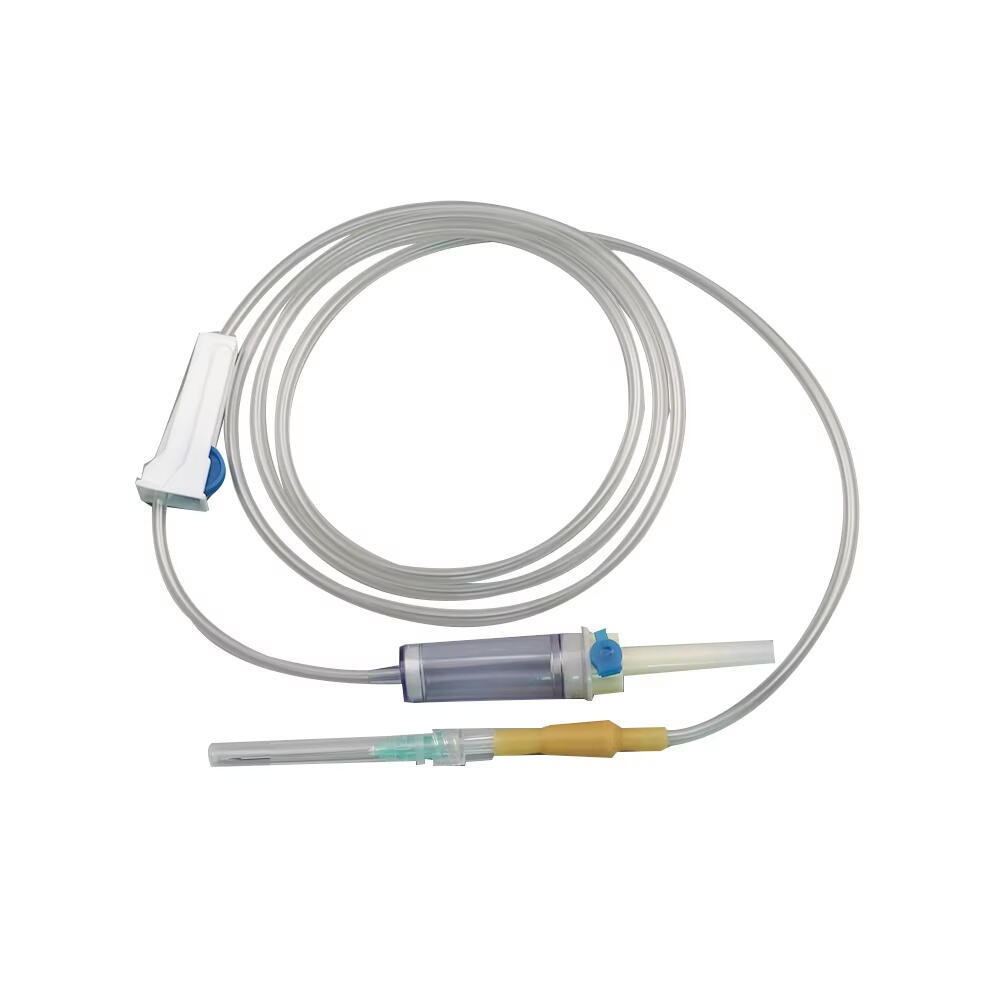
The 3 ml syringe no needle manufacturer stands at the forefront of medical device production, specializing in creating high-precision syringes that meet stringent healthcare standards. This manufacturer employs state-of-the-art automation technology and quality control systems to produce sterile, reliable syringes for various medical applications. The facility utilizes medical-grade materials, primarily polypropylene and polyethylene, ensuring biocompatibility and chemical resistance. The manufacturing process incorporates advanced molding techniques, automated assembly lines, and rigorous testing protocols to guarantee consistent product quality. These syringes feature precise graduation markings, smooth plunger action, and secure luer lock or luer slip connections, making them compatible with various needle types and medical devices. The manufacturer maintains ISO 13485 certification and follows GMP guidelines, demonstrating their commitment to quality and safety in medical device production. Their syringes find applications in healthcare settings, research laboratories, and pharmaceutical companies, serving various purposes from medication administration to sample collection.